
doi:10.2204/iodp.sp.310.2005
APPENDIX
ESO Sampling and Measurement Plan
This plan was discussed and agreed on during various meetings and subsequent communications with the chief scientists. Nevertheless, this plan is subject to amendment according to the scientific needs and interests of the Expedition Scientists or operational constraints.
Offshore Sampling and Analysis
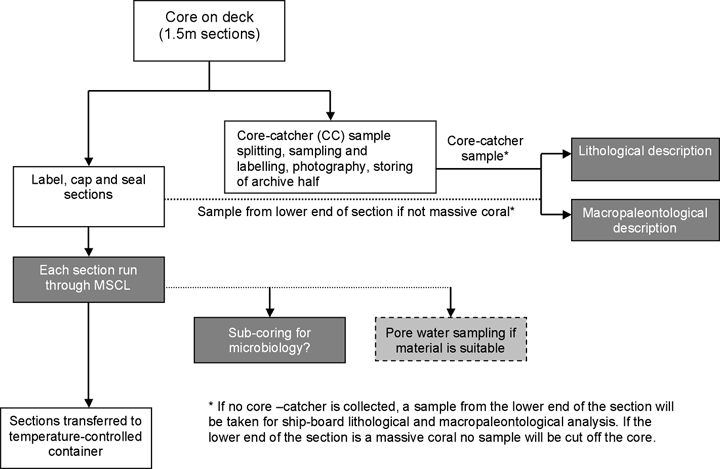
Core Curation
There will be a mobile core curation laboratory container onboard the drilling vessel, supervised by the Chief Curator. A second curator will cover the opposite shift. A sufficient number of core storage containers will be on the drilling vessel. There will be no splitting of the cores at sea, as it will be more efficient to carry out most of the following scientific analysis during an onshore party at Bremen.
As the cores will be collected in a plastic liner, the usual IODP curation procedures will be followed: the core will be cut on board into 1.5 m lengths and curated. It has been noted that it is important to store corals in dry conditions to avoid fungi and bacteria that may develop in coral skeletons, with the strong possibility of alteration of the initial geochemical signals.
Lithological and Macropaleontological Description
Core catcher samples will be collected, split, and labeled, and the working half handed over for lithological and macropaleontological description. If no core catcher is collected, a sample from the lower end of the section will be taken for shipboard lithological and macropaleontological analysis. If the lower end of the core is a massive coral, no sample will be cut off the core.
Inorganic Geochemistry
No major mud sequences are expected to be encountered at the proposed drill sites. However, if suitable material is recovered and there are requests from Expedition Scientists, pore water sampling (e.g., by centrifuge) may be conducted for fluid chemistry/circulation studies. In this case, pore water should be extracted immediately from a core sample, and ephemeral properties (e.g., salinity, alkalinity, and ammonia) will be analyzed right away. Depending on the parameter, the interstitial water sample might be specially treated in order to conserve it for later analyses.
Microbiology
Sampling
It is proposed that samples should be taken immediately in the field under the most sterile possible conditions. It will be important to know if microbes from the drilling fluids have entered the cavities during drilling. Ideally, fluorescent microspheres should be used during drilling, but these will not be used during Expedition 310 for environmental reasons. Results should be interpreted with care, as contamination may occur during drilling and any microbial material found may not be in situ. To limit the effects of contamination, samples will be washed with sterile seawater and only the attached microbes will be considered for further activity measurements (which includes typically >99% of the total biomass).
Fixing for Scanning Electron Microscopy/Energy Dispersive X-Ray Analysis/Light Microscopy Studies
It will be important to look for the biofilms in the field using a binocular microscope and a fluorescence microscope (i.e., to look for living microbes revealed by 4¢,6-diamindino-2-phenylindole [DAPI] staining). Also, counting should be done in the field (but no routine counting is required because we expect that the DAPI and acridine orange staining methods are probably difficult to apply in the coral reef environment because of unspecific binding of the dyes to carbonate minerals). The microbial community should be chemically fixed together with the mineral substrate using glutaraldehyde to preserve the primary structure, and the samples should be frozen at –80ûC to be transported back to the laboratory, where the microbial abundance (shipboard) and diversity (shore based) will be studied microscopically using staining techniques. Scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDAX) studies of microbial related carbonates (microbialites) will be made postcruise using the fixed samples.
Growth Studies
Appropriate growth media should be inoculated with selected core samples in the field. These will be returned to the laboratory, where the growth of microbes from samples will be studied postcruise. This living material can also be used for deoxyribonucleic acid (DNA) analysis.
Activity Measurements
One of the most sensitive tests to detect surface attached microbes (i.e., living cells) is the adenosine triphosphate (ATP) test with a luminometer and the firefly-based enzyme assay. The surfaces can be sampled with a specific type of swab, and the numerical results are available within seconds. This method is ideal for routine analysis, in comparison to the several hours required for the staining/counting methods (furthermore, we expect that the DAPI or acridine orange staining methods will probably be difficult to apply in this environment, see above).
Another sensitive test (qualitative and quantitative) for microbial activity is the measurement of microbial exoenzymes. We propose to measure alkaline phosphatase, glucosidase, and aminopeptidase exoenzymes as detected by fluorometry and compare the data with other subseafloor sites described in the literature. This test requires that defined amounts of sediment be incubated together with fluorescent dye-labeled substrates in stirred vials for 6–12 h and the released dye measured in a spectrofluorometer.
Offshore Petrophysics Measurements
Core Logging
Cores will be logged on the drilling vessel in a modified 20 ft container, housing a single MSCL track comprising one magnetic susceptibility loop and density, velocity, and resistivity sensors. The single core-logger system will include a full spares kit.
All the temperature-equilibrated core logging data acquired at sea will provide quality control/quality assurance checks when compared to repeat measurements planned for Bremen.
Downhole Logging
The following is a generic list of minimum and additional tools, based on formation properties discussed with proponents, and not on operator-based trademark names:
- Optical images: millimeter-scale geological description
- Acoustic images: centimeter-scale impedance and mesoscale porosity
- Spectral gamma logging: U, Th, K, and red algaes
- Acoustic velocity logging: VP and VS at 10–20 kHz
- Induction resistivity logging: pore fluid salinity and porosity
- Hydrochemical borehole fluid logging: pressure, temperature, pH, Eh, SP and fluid electrical conductivity to identify fluid circulation
Onshore Sampling and Analysis
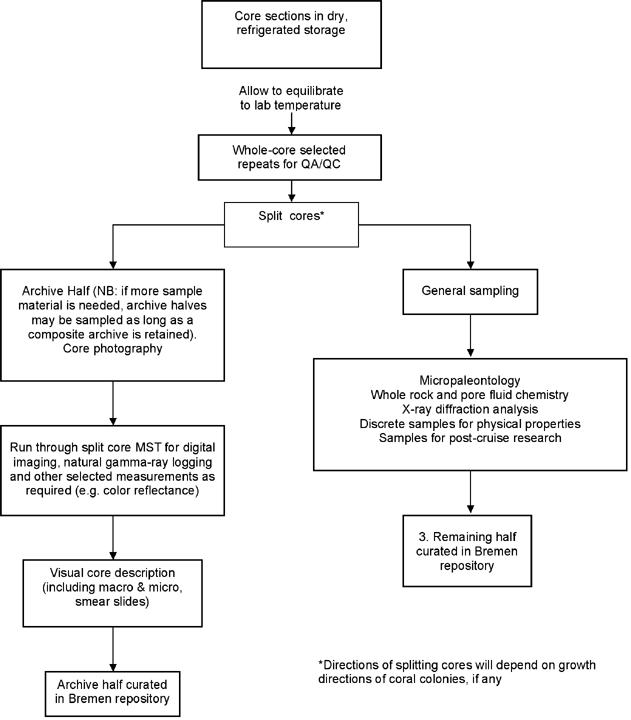
Location
After due consideration, it has been decided that there will be no splitting of the cores at sea. The sampling party will take place at the new IODP Core Repository and Laboratory at Bremen University in combination with access to the laboratories at the Department of Geosciences, the Research Center for Ocean Margins (RCOM), and the Centre for Marine Environmental Research (MARUM).
Planned Analysis and Available Facilities
The following facilities will be available for the Expedition Scientists at the IODP Bremen Core Repository. Note that it is not considered prudent to transport all these facilities to the island or onto a drilling vessel:
- Core splitting: an archive half will be set aside as per IODP policy.
- Core description: ESO is working in cooperation with the IODP U.S. Implementing Organization (USIO) to implement a system that is at least equivalent to the IODP/ODP standard. For data entry, ESO will employ an OffshoreDIS system that is entirely compatible with others being used in IODP.
- Core photography: core shots on a routine basis, close-ups on request.
- Core sampling: a detailed sampling plan will be devised at the completion of the offshore phase and after the scientists have submitted their revised sample requests.
- Thin section and smear slide preparation: description and interpretation.
- Micropalaeontology: microscope laboratory (with hood for sample preparation if acids needs to be applied).
- Inorganic geochemistry: whole-rock and pore fluid chemistry using inductively coupled plasma–mass spectrometry (ICP-MS) and X-ray fluorescence (XRF); carbonate and total organic carbon content using a LECO analyzer.
- X-ray diffraction analysis (XRD): bulk mineralogy (e.g., carbonate mineralogy, etc.).
- Petrophysical measurements
- Selected repeat whole-core measurements for QA/QC.
- Split-core multisensor core logger and natural gamma ray logging
- Physical properties of discrete samples (moisture and density [MAD]): determination of index properties (velocity, wet bulk density, grain density, porosity, and void ratio). Following IODP procedures, core samples will be oven-dried, the dried sample volume quantified using a five-chambered pycnometer, and masses measured using a high-precision balance.
- Color reflectance measurements: Minolta spectrophotometer.
- Digital line-scan camera on split-core multisensor core logger track.