Hall, I.R., Hemming, S.R., LeVay, L.J., and the Expedition 361 Scientists
Proceedings of the International Ocean Discovery Program Volume 361
publications.iodp.org
https://doi.org/10.14379/iodp.proc.361.204.2026
Data report: systematic taxonomy, morphology, and distribution of Late Neogene–Quaternary planktic foraminifera from the Agulhas Current region, International Ocean Discovery Program Expedition 361, Hole U1474A1
Vikram Pratap Singh,2 Rahul Dwivedi,2 and Shivani Pathak2
1 Singh, V.P., Dwivedi, R., and Pathak, S., 2026. Data report: systematic taxonomy, morphology, and distribution of Late Neogene–Quaternary planktic foraminifera from the Agulhas Current region, International Ocean Discovery Program Expedition 361, Hole U1474A. In Hall, I.R., Hemming, S.R., LeVay, L.J., and the Expedition 361 Scientists, South African Climates (Agulhas LGM Density Profile). Proceedings of the International Ocean Discovery Program, 361: College Station, TX (International Ocean Discovery Program). https://doi.org/10.14379/iodp.proc.361.204.2026
2 Department of Geology, Indira Gandhi National Tribal University, India. Correspondence author: vikram.singh@igntu.ac.in
Abstract
International Ocean Discovery Program Hole U1474A, drilled during Expedition 361 in the southwestern Indian Ocean, offers a high-quality archive for reconstructing Agulhas Current variability. The site yielded 104% core recovery over 256.11 m, spanning from the Late Miocene to recent. The sediments are exceptionally well preserved and contain a diverse planktic foraminiferal assemblage, including tropical, subtropical, temperate, and subpolar forms.
This study presents a detailed morphological and taxonomic analysis of Late Neogene–Quaternary planktic foraminifera from Hole U1474A, supported by scanning electron micrographs. Accurate species identification is essential for understanding mid-latitude paleoceanographic events and diachrony. The specimens exhibit minimal dissolution, with well-preserved wall textures and visible spines. A total of 63 species were identified, representing tropical to temperate faunas. Dominant genera include Globigerinoides, Globoconella, Globigerinita, and Neogloboquadrina. A semiquantitative distribution of these forms is also provided to support further biostratigraphic and paleoenvironmental reconstructions in the Agulhas Current region.
1. Introduction
The planktic foraminifera, which are excellent index fossils, form the backbone of the Cenozoic biostratigraphic and paleoceanographic studies. Because of their passive mode of life, they are susceptible to stresses caused by variations in water mass properties like temperature, salinity, and nutrient conditions. The response to changes in the ambient water mass conditions is recorded in the test of the planktic foraminifera, which is exploited for paleoceanographic and paleoclimatic studies. It therefore becomes prudent that these sensitive proxies be precisely identified to be used for paleoceanographic and paleoclimatic interpretations.
With the development of scanning electron microscopy (SEM), there has been a tremendous development in the taxonomic studies of planktic foraminifera over the last few decades. Several studies have been conducted on the taxonomic refinements of the Neogene–Quaternary planktic foraminifera (Lamb and Beard, 1972; Stainforth et al., 1975; Saito, 1977; Steineck and Fleisher, 1978; Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; Jenkins, 1985; Cifelli and Scott, 1986; Hemleben et al., 1989; Hilbrecht, 1996; Fox and Wade, 2013; Schiebel and Hemleben, 2017; Lam and Leckie, 2020a, 2020b; Brummer and Kučera, 2022). The integration of taxonomic studies on fossilized tests and molecular genetic studies on extant species of planktic foraminifera (e.g., Darling et al., 2006; André et al., 2013; Spezzaferri et al., 2015; Schiebel and Hemleben, 2017; Poole and Wade, 2019, and others) has opened new avenues to ascertain the affinity of various morphotypes.
The growing number of works and literature on the taxonomic revision of planktic foraminifera has sparked a debate on the selection of the appropriate nomenclature and the generic and specific assignment of planktic foraminifera. It warrants the development of a precise concept of the taxonomic identification of planktic foraminifera for use in studies pertaining to marine geology.
Another important characteristic of planktic foraminifera is latitudinal provincialism (Bé and Tolderlund, 1971), which controls their distribution according to the latitudes. It is an essential aspect of planktic foraminiferal research that assists in paleoceanographic and paleoclimatic reconstructions.
This work aims to present a detailed SEM examination of well-preserved planktic foraminiferal specimens from Hole U1474A, which helps to determine the taxonomy and morphological variability of 63 Late Miocene–recent species.
2. Materials and methods
2.1. Location and modern oceanography of the study area
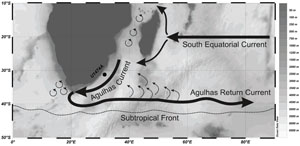
International Ocean Discovery Program (IODP) Hole U1474A was drilled during Expedition 361 in the northernmost part of the Natal Valley (31°13.00′S, 31°32.71′E) at a water depth of 3045 meters below sea level (mbsl), which is well above the carbonate compensation depth, resulting in good preservation of biogenic material (Figure F1; Hall et al., 2017). This site is located in the path of the Agulhas Current. It has the potential to record the variation in faunal assemblages due to the changes in the intensity of the current. The Agulhas Current is the largest western boundary current (Simon et al., 2013) that affects the surface dynamics in the Indian Ocean. The warm water of the Agulhas Current transported to the Atlantic Ocean via the Indo-Atlantic gateway by the Agulhas leakage in the form of eddies and rings (Lutjeharms, 2006) has a significant impact on the returning arm of the Atlantic Meridional Overturning Circulation. After 40°S, the Agulhas Current retroflects and returns to the Indian Ocean as the Agulhas Return Current, which flows along the Subtropical Front (Lutjeharms, 2009). The Subtropical Front is a hydrodynamic front that is the "gatekeeper" south of Africa (Graham and De Boer, 2013). It changes its position with variation in the climate, with a more northward position indicative of glacial climate and a southward position marking a return to warmer conditions (Peeters et al., 2004; Caley et al., 2014; Singh et al., 2023). The northward position of the Subtropical Front allows the ingression of cold temperate waters into the relatively warmer subtropical latitude, thereby causing a shift in the ecotones and changing the faunal assemblages to be dominated by characteristic cold-water forms. In the present work, we observed several episodes of significant rise in the relative abundance of cold-water forms, dominated by the genus Globoconella. Thus, the variation in the planktic foraminiferal assemblage is an important proxy for paleoceanographic reconstruction.
The terrigenous part of the sediment core recovered from Hole U1474A is clay dominated, and the biogenic fraction of the sediment is composed primarily of calcareous nannofossils, foraminifera, and siliceous sponge spicules, which makes it foram-nanno ooze (Hall et al., 2017).
The total length of the cored section is 254.1 m, with core recovery of 104% in 29 advanced piston cores (Hall et al., 2017). The dominant lithology consists of yellow to greenish gray clay that contains foraminifera and nannofossils, with occasional occurrences of sand and turbidite lenses. Total biogenic carbonate concentration is estimated to be around 39% (Hall et al., 2017). Of the total cored length, we used 233.4 m, comprising 25 cores for the present work (361-U1474A-1H through 25H), from which the sediments were sampled at an interval of 30 cm. The volume of each sample was 10 cm3, and the approximate temporal gap between the two samples was calculated to be 6–8 ky (Singh et al., 2023). A total of 710 samples, spanning Late Miocene to recent, were analyzed for planktic foraminiferal assemblages to establish Late Neogene–Quaternary biostratigraphy and paleoceanography in the Agulhas Current region.
2.2. Sample processing using the wet-sieving technique
In this study, planktic foraminiferal assemblages from Hole U1474A core samples obtained from Kochi Core Center (Japan) were studied. A detailed biostratigraphy was established (Singh et al., 2025) for the 710 samples at 30 cm sampling intervals, spanning the last ~7 My. Additionally, planktic foraminiferal census count and stable isotope data for these samples were generated for the Late Neogene–Quaternary paleoceanographic and paleoclimatic reconstruction.
The samples were oven-dried overnight at approximately 60°C and weighed. After drying, they were dissolved in 1 L of alkaline solution containing 1 g sodium hexametaphosphate (NaPO3)6 and 5 g sodium hydroxide (NaOH). To assist the disintegration of the clay, occasionally 10 mL H2O2 was added. The solution was then subjected to an ultrasonic bath for 2 min to enhance disintegration. After this treatment, the samples were thoroughly washed with tap water over sieves of two sizes: ≥150 µm and ≥100 µm. The samples were left to dry at room temperature overnight. The dried residue of the two sizes was carefully transferred into three tubes that corresponded to 150 µm, 100 µm, and the base for mud collection for each sample.
2.3. Age model for Hole U1474A
A detailed paleomagnetic record was obtained from the shipboard investigation (Hall et al., 2017) for Hole U1474A. Table T1 lists the paleomagnetic events recorded for Hole U1474A along with their absolute ages, as derived from Ogg (2020).
This record was used to calculate the rate of sedimentation and estimate the approximate age of the samples under investigation, assuming a uniform rate of sedimentation. The rate of sedimentation was determined by dividing the depth of the core by the corresponding paleomagnetic age and was then multiplied by the depth of the samples to estimate their ages.
3. Results
3.1. Foraminifera
The planktic foraminiferal assemblages from Hole U1474A spanning Late Neogene–Quaternary were analyzed for the taxonomic studies. We encountered 63 species belonging to 21 genera that were prominent in their occurrence in Hole U1474A. The list of the genera and species is provided in Table T2.
The processed samples were spread in a picking tray and were studied under the Zeiss Discovery V.8 Stereozoom microscope with camera facility. The planktic foraminifera were identified to the species level following the taxonomic work of Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Huber et al. (2016), and Lam and Leckie (2020a).
The details of surface ultrastructure, pores, spines, keel, and so on were studied using a Carl Zeiss EVO-18 scanning electron microscope at the Department of Geology at Banaras Hindu University (India). The specimens were mounted on stubs using carbon tape and were coated with gold-palladium alloy before being loaded in the SEM.
3.2. Diversity
The diversity of foraminiferal assemblages in Hole U1474A is quite high. We identified a total of 63 species from Late Miocene to recent comprising a mixture of warm tropical–subtropical and cool temperate–subpolar forms. Although the warm-water dwellers were dominant forms in the entire core, the Quaternary section showed an unprecedented rise in the cold-water species, which occasionally comprised more than half of the entire assemblage of planktic foraminifera.
3.3. Biostratigraphy
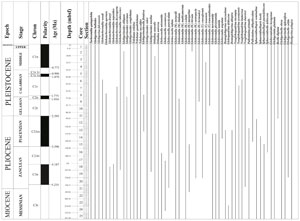
Hall et al. (2017) conducted low-resolution biostratigraphy aboard ship for Hole U1474A using core catcher samples supplemented with an additional one sample per section for the entire core. Although this study was preliminary and used Wade et al. (2011) as the reference for the ages, we revised the entire shipboard range charts and updated the biostratigraphy following Kennett (1973), Jenkins and Srinivasan (1986), and Lam and Leckie (2020b). The detailed biostratigraphy is presented in Singh et al. (2025). The stratigraphic range of the important planktic foraminiferal species is given in Figure F2.
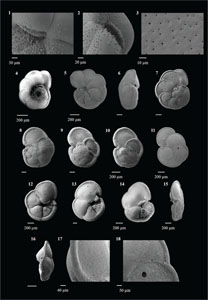
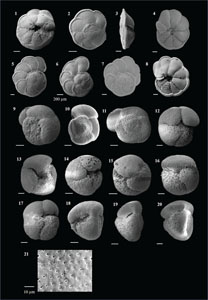
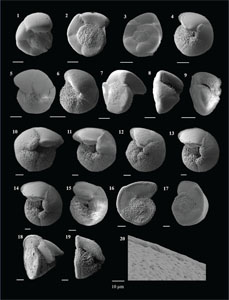
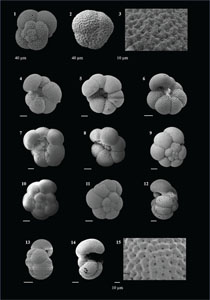
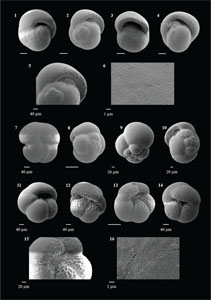
3.4. Systematic paleontology
The systematic descriptions of the encountered species follow the existing understanding of Late Neogene–Quaternary planktic foraminiferal taxonomy as well as new taxonomic concepts developed and revised over the years (Kennett and Srinivasan, 1983; Bolli and Saunders, 1985, Schiebel and Hemleben, 2017; Lam and Leckie, 2020a; Brummer and Kučera, 2022).
In this study, we have documented as many species as possible encountered from the Agulhas Current region to prepare a catalogue of the tropical and temperate forms from the mid-latitudes in the southwest Indian Ocean. We have elaborated the significant morphological features for each species to help with precise identification in each Remarks section. Although we tried to incorporate the minute and subtle observations used for identification, we do not claim that these are the complete descriptions of any species. These observations may be used along with the relevant literature for taxonomic identifications (e.g., Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; Cifelli and Scott, 1986; Scott et al., 1990; Schiebel and Hemleben, 2017; Wade et al. 2018; Lam and Leckie, 2020a; Brummer and Kučera, 2022). Along with the published work, we also cite the website https://www.mikrotax.org/pforams (Huber et al., 2016) for latest concepts, SEM images, and references.
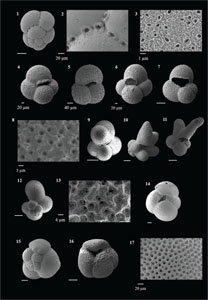
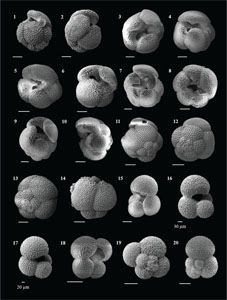
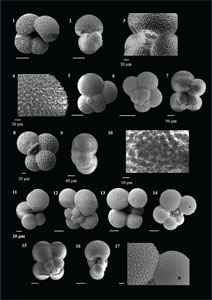
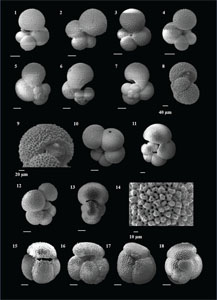
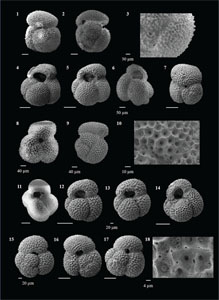
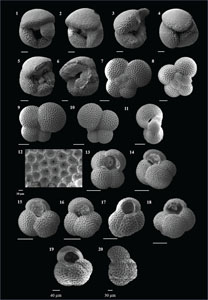
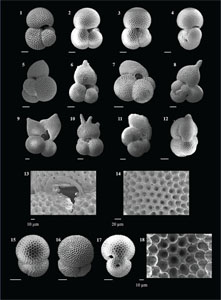
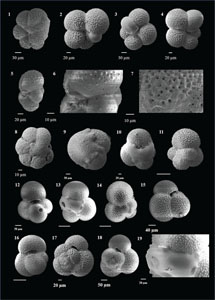
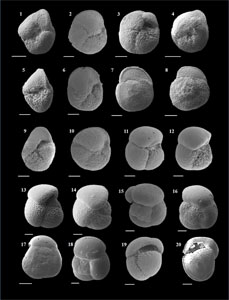
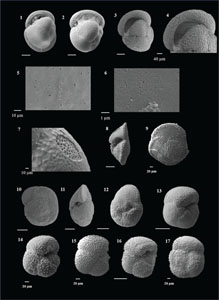
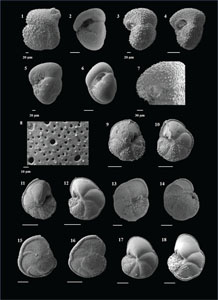
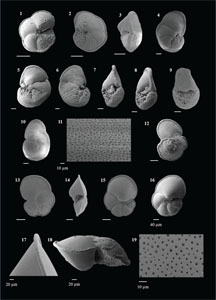
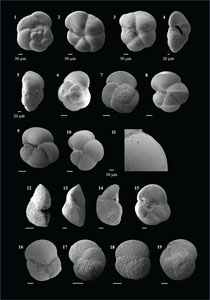
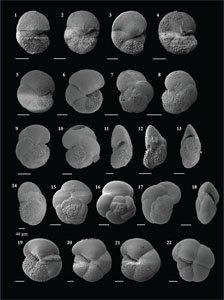
The species that were excluded from the list are those with extremely rare occurrences in Hole U1474A. The basionyms, synonyms (if any), and original references for each species are included in the notes that follow. SEM images of specimens are designed to capture the range of morphological variability within a species concept.
The plates are arranged by the family in which the species occur, within which the taxa are listed alphabetically by genus and species name. The images of various morphotypes within a species are arranged in the stratigraphic order of occurrence with the core.
Order FORAMINIFERIDA d'Orbigny 1826
Superfamily GLOBIGERINOIDEA Carpenter, Parker and Jones 1862
Family CANDEINIDAE Cushman 1927
Type species Candeina nitida d'Orbigny 1839
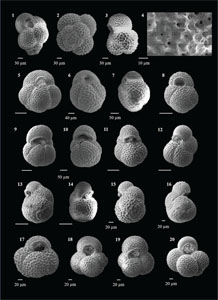
Candeina nitida (d'Orbigny 1839)
(Plate P1, figures 1–3)
Synonym: Candeina milletti Dolfus (1905), Candeina nitida praenitida Blow (1969)
Type species: Candeina nitida d'Orbigny, 1839
References: d'Orbigny (1839), Kennett and Srinivasan (1983), Loeblich and Tappan (1994), Norris (1998), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Meilland et al. (2022), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-23H-7, 54–56 cm, to 1H-1, 0–2 cm
Remarks. C. nitida is an extant species characterized by a high trochospiral compact test. The final whorl has three chambers. The surface is smooth and microperforate. The characteristic feature of C. nitida is the presence of sutural supplementary apertures, each of which is bordered by a rim.
This thermocline dweller (Lessa, 2020) is found in tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). This species is rare and shows irregular occurrence in Hole U1474A.
Family GLOBIGERINIDAE Carpenter, Parker and Jones 1862
Genus Beella Banner and Blow 1960
Type species Globigerina digitata Brady 1879
Beella praedigitata (Parker 1967)
(Plate P1, figures 4–8)
Basionym: Globigerina praedigitata
Synonym: Beella megastoma Earland (1934)
Type species: Beella praedigitata (Parker, 1967)
References: Parker (1967), Kennett (1973), Kennett and Srinivasan (1983), Lam and Leckie (2020a).
Observed stratigraphic range: 361-U1474A-23H-7, 54–56 cm, to 4H-1, 10–12 cm
Remarks. B. praedigitata has a low trochospiral test with four to five inflated chambers in the final whorl. The surface is spinose and smooth (Aze et al., 2011), consisting of circular pores and tubercles representing spine bases (Kennett and Srinivasan, 1983).
It evolved from G. bulloides in the Late Miocene (Kennett and Srinivasan, 1983) and evolved into B. digitata by giving rise to radially elongate chambers (Kennett and Srinivasan, 1983; Lam and Leckie, 2020a). B. praedigitata is a thermocline dweller (Aze et al., 2011) extending from tropical to temperate latitudes (Kennett and Srinivasan, 1983). It is extremely rare in Hole U1474A.
(Plate P1, figures 9–13)
Basionym: Globigerina digitata
Synonyms: Beella chathamensis McCulloch (1977), Beella guadalupensis McCulloch (1977), Hastigerina frailensis McCulloch (1977).
Type species: Beella digitata Brady, 1879
References: Brady (1879), Kennett (1973), Kennett and Vella (1975), Kennett and Srinivasan (1983), Lam and Leckie (2020a), Brummer and Kučera (2022).
Observed stratigraphic range: 361-U1474A-11H-7, 35–37 cm, to 1H-1, 0–2 cm
Remarks. B. digitata differs from B. praedigitata in having radially elongate chambers. It has a high trochospiral test, with four to five radially elongated chambers in the final whorl. The wall is spinose and irregularly cancellate (Aze et al., 2011).
B. digitata is a thermocline dweller (Aze et al., 2011) extending from tropical to temperate latitudes (Kennett and Srinivasan, 1983). It is a regularly occurring species in the Quaternary samples but very low in abundance.
Genus Dentoglobigerina Blow 1979
Type species Globigerina galavisi Bermúdez 1961
Dentoglobigerina venezuelana (Hedberg 1937)
(Plate P1, figures 14–17)
Basionym: Globigerina venezuelana
Synonym: Globoquadrina venezuelana, Globoquadrina conglomerata (?)
Type species: Dentoglobigerina venezuelana Hedberg, 1937
References: Hedberg (1937), Postuma (1971), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Wade et al. (2018), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 13H-4, 77–79 cm
Remarks. D. venezuelana is characterized by a very large test with a wide umbilicus. There are four reniform chambers in the final whorl, and the wall is spinose to cancellate (Wade et al., 2018). The test is sometimes pustulose on the umbilical shoulders and may also show the presence of umbilical teeth, thereby closely resembling Dentoglobigerina altispira.
There has been considerable debate over the generic affinity of this species. Kennett and Srinivasan (1983) assigned it to Globoquadrina, which was also accepted by Bolli and Saunders (1985) and Aze et al. (2011). Later, Wade et al. (2018) determined that this species was spinose and thus transferred it to Dentoglobigerina. Lam and Leckie (2020a) have also included it in Dentoglobigerina. Another form, Globoquadrina conglomerata Schwager (1866) was considered a distinct species by Saito et al. (1981), Hemleben et al. (1989), and Lam and Leckie (2020a). Lam and Leckie (2020a) suggest that it is the extant form and a separate species that differs morphologically from D. venezuelana. Banner and Blow (1960), Parker (1962), and later Wade et al. (2018) considered G. conglomerata a synonym of D. venezuelana. Brummer and Kučera (2022) chose to retain venezuelana in Globoquadrina based on the genetic data by Morard et al. (2019), which does not show any affinity to the spinose clade. This thermocline dweller is globally found in low and mid latitudes (Wade et al., 2018). In Hole U1474A, it is a commonly occurring species during the Late Pliocene, although the abundance is quite low.
Dentoglobigerina altispira (Cushman and Jarvis 1936)
(Plate P2, figures 1–14)
Basionym: Globigerina altispira
Synonym: Dentoglobigerina altispira altispira, Dentoglobigerina altispira conica
Type species: Dentoglobigerina altispira Cushman and Jarvis, 1936
References: Cushman and Jarvis (1936), Postuma (1971), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Fox and Wade (2013), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 13H-4, 77–79 cm
Remarks. D. altispira is characterized by large, high trochospiral tests with four to five chambers in the final whorl. The chambers are appressed toward the umbilicus. The umbilicus is wide open and deep and shows umbilical teeth. The surface is cancellate with pores in pore pits.
Another species, D. altispira globosa Bolli (1957), differs from D. altispira in having five to six chambers in the final whorl and a low trochospiral test (Kennett and Srinivasan, 1983). It has not been differentiated in this work owing to the scope of the objectives.
D. altispira is a shallow mixed-layer dweller (Srinivasan and Sinha, 2000; Aze et al., 2011) and is commonly found in tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). It is a regularly occurring species in Hole U1474A in samples spanning the Pliocene and shows moderately high abundance, occasionally reaching up to 6%.
Genus Globigerina d'Orbigny 1826
Type species Globigerina bulloides d'Orbigny 1826
Globigerina bulloides (d'Orbigny 1826)
(Plate P2, figures 15–20; Plate P3, figures 1–4)
Basionym: Globigerina bulloides
Type species: Globigerina bulloides d'Orbigny 1826
References: d'Orbigny (1826), Schwager (1866), Banner and Blow (1960), Lamb and Beard (1972), Kennett and Srinivasan (1983), Jenkins (1985), Shrivastav et al. (2016), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. bulloides is a commonly occurring species in the eutrophic waters along the Equator, temperate regions, and in the-low latitude upwelling zones (Bé, 1977; Duplessy et al., 1981; Naidu et al., 1992; Naidu and Malmgren, 1996; Sinha et al., 2006; Shrivastav et al., 2016; Schiebel and Hemleben, 2017; Brummer and Kučera, 2022). It is characterized by a low trochospiral test with a characteristic G. bulloides-type wall texture. This wall texture is characterized by a hispid surface (Kennett and Srinivasan, 1983), which has thin and round spines, and an irregular pore pattern with a narrow space between pores (Hemleben and Olsson, 2006). The overall test morphology closely resembles quite a few other species like Globigerinella calida, Globigerinella obesa, Globigerina falconensis, and sometimes vaguely Globigerinita glutinata (without bulla). However, the umbilical aperture in G. bulloides, which is slightly eccentric, distinguishes it from G. calida, which has the aperture facing either left or right side (Schiebel and Hemleben, 2017); G. obesa differs from G. bulloides by its extraumbilical aperture (Shrivastav et al., 2016); the absence of apertural rim or lip separates it from G. falconensis (Kennett and Srinivasan, 1983); and the wide, high-arch aperture and surface ultrastructure distinguish G. bulloides from G. glutinata (Darling and Wade, 2008). The test of G. bulloides frequently exhibits the presence of a kummerform final chamber.
The considerable variability in the shape and number of chambers in G. bulloides has led to great confusion in taxonomic identification (Lamb and Beard, 1972) and the erection of several species by other authors, for example, Globigerina bermudezi Seiglie 1963, Globigerina cariacoensis Rögl and Bolli 1973, Globigerina megastoma Earland 1934, Globigerina quadrilatera Galloway and Wissler 1927, and Globigerina riveroae Bolli and Bermúdez 1965, all of which are morphotypes and closely resemble G. bulloides (Shrivastav et al., 2016). Kennett and Srinivasan (1983) dubbed these morphotypes phenotypic variants of G. bulloides.
The molecular genetic studies by various authors have distinguished 14 genetic types of G. bulloides (Darling et al., 2000; Darling and Wade, 2008; André et al., 2013; Morard et al., 2013; Schiebel and Hemleben, 2017). Later, André et al. (2014) suggested that only seven genotypes of G. bulloides qualify for a species status. Seears et al. (2012) showed bipolar distribution of a few genotypes of G. bulloides, suggesting a gene flow across the tropics, as suggested earlier by Darling et al. (2000). This species has attracted significant attention for its correct taxonomic identification owing to its importance as proxy for upwelling episodes in the low and mid latitudes.
This species is a temperate-latitude, mixed-layer dweller (Bé and Tolderlund, 1971) and thrives in high nutrient conditions, indicative of upwelling in lower latitudes (Thiede, 1975; Bé and Hutson, 1977; Naidu and Malmgren, 1996; Seears et al., 2012; Shrivastav et al., 2016; Schiebel and Hemleben, 2017) and seasonally enhanced primary production at mid and high latitudes (Bé and Tolderlund, 1971; Ottens, 1992; Chapman, 2010; Schiebel and Hemleben, 2017).
In the present study, this species is encountered throughout the Late Neogene–Quaternary span, with significantly high abundance in several samples representing episodes of enhanced productivity in Hole U1474A.
Globigerina falconensis (Blow 1959)
(Plate P3, figures 5–10)
Basionym: Globigerina falconensis
Synonym: Globorotalia (Turborotalia) palpebra Brönnimann and Resig (1971)
Type species: Globigerina falconensis Blow, 1959
References: Blow (1959), Kennett and Srinivasan (1983), Jenkins (1985), Iaccarino (1985), Shrivastav et al. (2016), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022), Fabbrini et al. (2023)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. The test of G. falconensis closely resembles G. bulloides in its appearance, with G. bulloides-type wall structure, globular chambers, and the slightly eccentric umbilical aperture. The main difference lies in the presence of a distinct apertural lip. Although the apertural area is usually smaller than G. bulloides (Schiebel and Hemleben, 2017), it is not very distinct unless the test is morphometrically analyzed. Kennett and Srinivasan (1983) mentioned that the final chamber of G. falconensis is distinctly smaller than the penultimate chamber, but this feature was not observed in all the specimens. There were several forms that did not adhere to this observation by Kennett and Srinivasan (1983). Thus, the presence of lip in the last chamber wall becomes an essential criterion for its distinction.
Fabbrini et al. (2023), on the basis of morphometric analysis, reported the presence of interpore ridges in G. falconensis and opined that the wall structure was more pseudocancellate than G. bulloides-type. Fabbrini et al. (2023) described a new morphotype, Globigerina neofalconensis, distinguished from G. falconensis by its more lobate profile, more loosely coiled test, and wider umbilicus.
In the present study, all morphotypes with lobate outline, globular chambers, G. bulloides-type wall, and aperture with a prominent lip have been identified as G. falconensis. It is a commonly occurring species in Hole U1474A, but its abundance has mostly stayed within 5%.
Genus Globigerinella Cushman 1927
Type species Globigerina aequilateralis Brady 1879
Globigerinella calida (Parker 1962)
(Plate P3, figures 11–17)
Synonym: Globigerina calida calida, Bolliella calida calida
Type species: Globigerina calida Parker, 1962
References: Parker (1962), Kennett and Srinivasan (1983), Chaproniere et al. (1994), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-19H-7, 47–49 cm, to 1H-1, 0–2 cm
Remarks. G. calida has a smaller, less planispiral test, which separates it from G. siphonifera, and the radially elongated chambers in the final whorl distinguish it from G. obesa (Brummer and Kučera, 2022). It has a G. bulloides-type wall, characteristic of this lineage.
This thermocline dweller is commonly found in tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011). In Hole U1474A, G. calida occurs regularly with higher abundance in Quaternary samples than Pliocene samples.
Globigerinella obesa (Bolli 1957)
(Plate P4, figures 1–9)
Synonym: Globigerina praebulloides Blow (1959)
Type species: Globigerinella obesa Bolli, 1957
References: Bolli (1957), Kennett (1973), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Spezzaferri et al. (2018), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 32–34 cm
Remarks. G. obesa is characterized by a strongly lobulate test with four chambers in the final whorl. The surface is densely perforated and spinose with spine bases coalescing into regular ridges (Kennett and Srinivasan, 1983).
G. obesa very closely resembles G. bulloides, but it can be distinguished on the basis of flat spiral side and extraumbilical aperture (Lam and Leckie, 2020a). It is commonly found in low to mid latitudes (Spezzaferri et al., 2018). In Hole U1474A, it is extremely low in abundance.
Globigerinella siphonifera (d'Orbigny 1839)
(Plate P4, figures 10–14)
Basionym: Globigerina siphonifera
Synonym: Globigerina aequilateralis Brady (1879), Globigerina aequilateralis involuta Cushman (1917), Hastigerina aequilateralis
References: d'Orbigny (1839), Brady (1879), Kennett (1973), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Pearson (1995), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. siphonifera is characterized by an irregular planispiral test. Five to six chambers are present in the final whorl. The surface is spinose with G. bulloides-type wall and rounded spines. The aperture is wide arch and extends from umbilicus to spiral side, without rim or lip.
G. siphonifera is a thermocline dweller, living in low to mid latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011). It occurs regularly in Hole U1474A in moderate abundance.
Genus Globigerinoides Cushman 1927
Type species Globigerina rubra d'Orbigny 1839
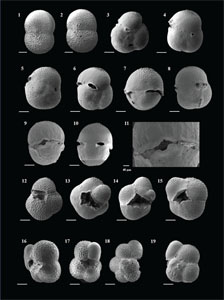
Globigerinoides conglobatus (Brady 1879)
(Plate P4, figures 15–18; Plate P5, figures 1–3)
Basionym: Globigerina conglobatus
Synonym: Globigerinoides carimanensis Bermúdez (1960)
Type species: Globigerinoides conglobatus Brady, 1879
References: Brady (1879), Banner and Blow (1960), Jenkins (1971), Kennett (1973), Fleisher (1974), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. conglobatus is characterized by its compact, large test with three to four flattened chambers, giving a subquadrate outline to the test. It can be distinguished by its tight coiling and an unusually flat final chamber. The test is often very thick and has a G. ruber-type wall. The surface has large pores, triangular spines and spine holes, which are often obscured by a thick calcite crust (Schiebel and Hemleben, 2017). The umbilicus is small, and the primary aperture varies from a low slit to a low arch centered on the previous chambers. The spiral side bears two or more sutural supplementary apertures. An interesting observation in the present work was a higher abundance of morphotypes with three chambers during the Late Miocene–Early Pliocene, whereas four-chambered forms dominated Late Pliocene–Quaternary. G. conglobatus is an extant species that prefers open ocean mixed-layer habitat (Aze et al., 2011) and extends from tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983; Schiebel and Hemleben, 2017) and is often transported by ocean currents to lower mid latitudes (Schmuker and Schiebel, 2002).
This species is found across Late Neogene–Quaternary and rarely exceeds 1.5% abundance in Hole U1474A.
Globigerinoides extremus (Bolli and Bermúdez 1965)
(Plate P5, figures 4–10)
Basionym: Globigerinoides obliquus extremus
Type species: Globigerinoides extremus Bolli and Bermúdez, 1965
References: Bolli and Bermúdez (1965), Postuma (1971), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 8H-6, 4–6 cm
Remarks. G. extremus evolved from G. obliquus by the development of laterally compressed four chambers in the final whorl and distinctly flattened final chamber (Kennett and Srinivasan, 1983; Bolli and Saunders, 1985), resembling a beret (Lam and Leckie, 2020a). The surface is distinctly pitted, and has G. ruber/T. sacculifer-type wall. The surface exhibits tubercles with spine holes, which were formed when the spine was shed. The primary aperture is oblique in shape, umbilical, and there is one supplementary aperture opposite the primary one.
G. extremus extends from tropical to cool subtropical latitudes (Kennett and Srinivasan, 1983), preferring open ocean mixed-layer habitat (Aze et al., 2011). It occurs in extremely low abundance in the upper part of the core compared to the lower part, where it is common to abundant.
Globigerinoides obliquus (Bolli 1957)
(Plate P5, figures 11–18)
Basionym: Globigerinoides obliqua
Type species: Globigerinoides obliqua Bolli (1957)
References: Bolli (1957), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Spezzaferri (1994), Lam and Leckie (2020a), Singh et al. (2021)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 2H-3, 52–54 cm
Remarks. G. obliquus shows a lobate test with four ovate chambers in the final whorl, and the last chamber is obliquely compressed. The surface is spinose, distinctly pitted and perforate, exhibiting G. ruber/T. sacculifer-type wall (Spezzaferri et al., 2018). The primary aperture is umbilical, high and wide arch. There is one supplementary aperture on the spiral side, opposite the primary aperture.
This cosmopolitan species occurs commonly at middle and high latitudes (Spezzaferri et al., 2018) and prefers mixed-layer habitat (Chaisson and Ravelo, 1997).
The lowest occurrence (LO) of G. obliquus is an important biostratigraphic event and has been assigned a mid-Pleistocene age by several authors (Kennett and Srinivasan, 1983; Aze et al., 2011; Wade et al., 2011; Brummer and Kučera, 2022) and Late Pleistocene by others (Sinha and Singh, 2008, 2022; Lam and Leckie, 2020b; Kaushik et al., 2020). In the present study, the LO of this species was found in the samples of the Late Quaternary. In Hole U1474A, G. obliquus occurs in high abundance in the lower part of the core spanning the Pliocene, and the Quaternary section has extremely low abundance.
Globigerinoides ruber (d'Orbigny 1839)
(Plate P6, figures 1–10)
Type species: Globigerinoides ruber d'Orbigny (1839)
References: d'Orbigny (1839), Banner and Blow (1960), Postuma (1971), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Loeblich and Tappan (1994), Wang (2000), Numberger et al. (2009), Spezzaferri et al. (2015), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Jayan et al. (2021), Latas et al. (2023)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. ruber can be distinguished by a three-chambered, low to high trochospiral test with its primary and supplementary sutures symmetrically placed above the suture between the earlier chambers of the final whorl. The suture line seems to bisect the primary aperture. The surface is spinose with a G. ruber-type wall.
This species exhibits several morphotypes during its entire stratigraphic range, especially during the Quaternary, during which it shows a wide range of variation in the height of the spire and tightness of the test coiling (Kennett and Srinivasan, 1983). These variations led to the recognition of several taxa: forms with a high trochospiral test were named Globigerinoides pyramidalis (Van den Broeck, 1876), forms with tightly coiled tests were named Globigerinoides elongatus (d'Orbigny, 1826), and those with a compact test and smaller aperture were called Globigerinoides cyclostomus (Galloway and Wissler, 1927). All these forms were considered phenotypic variants by Kennett and Srinivasan (1983).
Two chromotypes of G. ruber were identified as phenotypes: a white variety, also referred to as G. ruber subspecies albus (Morard et al., 2019), and a pink variety, G. ruber subspecies ruber (d'Orbigny, 1839). The name of this species is actually derived from the pink color of its tests (Aurahs et al., 2009; Morard et al., 2019). The white variety was originally given the name G. ruber forma albus by Boltovskoy (1968) and was later differentiated into G. ruber sensu stricto (s.s.) and G. ruber sensu lato (s.l.) by Wang (2000) on the basis of their morphometry and stable isotopic compositions.
The white variety of G. ruber is extant in all the ocean basins and dominates in the tropical and subtropical water masses (Bé and Hamlin, 1967; Hemleben et al., 1989), and the pink variety of G. ruber became extinct in the Indian and Pacific Oceans during Late Pleistocene (Schiebel and Hemleben, 2017) and preferred warmer habitats than white variants (Bé and Hamlin, 1967, Hemleben et al.1989). The LO of G. ruber (pink) is an important biostratigraphic event in the Indian and Pacific oceans (Wade et al., 2011). Numberger et al. (2009) recognized four morphotypes of G. ruber (white) on the basis of morphometric analysis and named them "type a or normal, type b or platys, type c or elongate, and type d or kummerform."
G. ruber is an important species inhabiting the top mixed layer (Aze et al., 2011). It exhibits oligotrophic behavior and serves as an important proxy for the thickness of the mixed layer (Sinha et al., 2006). This species has been effectively used for the stable oxygen isotope as well as Mg/Ca ratios for reconstruction of past sea-surface temperatures. G. ruber is a characteristic species for the Agulhas Current and constitutes 40%–60% of the modern Agulhas fauna (Simon et al., 2013). The average abundance of G. ruber in Hole U1474A is higher during the Late Pliocene to recent and rare during the Early Pliocene.
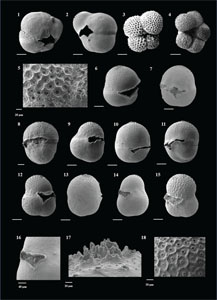
Globigerinoides tenellus (Parker 1958)
(Plate P6, figures 11–14)
Type species: Globigerinoides tenella Parker (1958)
Reference: Parker (1958), Frerichs (1971), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-16H-5, 77–79 cm, to 1H-1, 0–2 cm
Remarks. G. tenellus can be distinguished by its small, compact test with spinose and cancellate wall (Kennett and Srinivasa, 1983; Aze et al., 2011). The primary aperture is large and circular, with a distinct rim, and placed centrally. It has one supplementary aperture on the spiral side. This species closely resembles Globoturborotalita rubescens, but for its supplementary aperture. In many modern analyses it was included in Globoturborotalita despite having a supplementary aperture (Schiebel and Hemleben, 2017). Kennett and Srinivasan (1983) included this species in Globigerinoides and considered G. rubescens as the ancestor. Morard et al. (2019), on the basis of molecular genetics, included it in Globigerinoides and assigned G. elongatus as its ancestor.
G. tenellus can be distinguished from G. rubescens by the presence of a supplementary aperture. It is a mixed-layer dweller (Aze et al., 2011), present in tropical to temperate latitudes (Kennett and Srinivasan, 1983). In the present study, this species has occurred from Late Pliocene to recent, but is quite low in abundance.
Genus Globigerinoidesella El-Naggar 1971
Type species Globigerina fistulosa Schubert 1910
Globigerinoidesella fistulosa (Schubert 1910)
(Plate P6, figures 15–20)
Basionym: Globigerina fistulosa
Synonyms: Globigerinoides fistulosus, Globigerinoides sacculifera fistulosus, Globigerinoides quadrilobatus hystricosus
Type species: Globigerinoidesella fistulosa Schubert (1910)
References: Schubert (1910), Parker (1967), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Poole and Wade (2019)
Observed stratigraphic range: 361-U1474A-13H-5, 87–89 cm, to 8H-4, 77–79 cm
Remarks. G. fistulosa differs from its ancestor T. sacculifer (Kennett and Srinivasan, 1983; Spezzaferri et al., 2015) in having a large test and fistulose proturbances on the chambers in the final whorl. It has a cancellate surface and differs from a T. sacculifer-type wall in being spinose (Aze et al., 2011). The primary aperture is large, rimmed, and interiomarginal, and there are multiple supplementary sutural apertures on the spiral side.
G. fistulosa is considered to be of great importance in biostratigraphic studies. The LO event of G. fistulosa has been used to mark the top of the Olduvai Normal Event (Srinivasan and Sinha, 1991, 1992; Berggren et al., 1995). Earlier, this event was also used to demarcate the Pliocene/Pleistocene boundary (Berggren et al., 1995; Sinha and Singh, 2008) when the age of the boundary was considered to be 1.8 Ma (Gradstein et al., 2004). After the age of the Pliocene/Pleistocene boundary was lowered to 2.588 Ma (Hilgen et al., 2009; Gibbard et al., 2010; Raffi et al., 2020), this event lost its utility as the boundary marker. This event is still utilized to identify the Gelasian/Calabrian boundary in the sedimentary record. The LO of G. fistulosa has also been used to mark the base of Zone PT1 by Wade et al. (2011), and Singh and Sinha (2022) identified this event close to the Olduvai base in Ocean Drilling Program Hole 762B.
G. fistulosa exhibits several morphotypes. Kennett and Srinivasan (1983) considered the forms with elongated digitations on the last few chambers in the final whorl as G. fistulosa. Belford (1962) erected a new subspecies, Globigerinoides quadrilobatus hystricosus, for the forms that had elongated final chambers with digitations. It was considered a primitive phylogenetically related form to G. fistulosa (Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; Poole and Wade, 2019). Bolli and Saunders (1985) identified the forms resembling G. fistulosa as Globigerinoides trilobus fistulosus, Globigerinoides trilobus cf. fistulosus, and Globigerinoides trilobus A on the basis of the extension of peripheral ends of the last few chambers in fistule-like projections.
The generic assignment of G. fistulosa also underwent revision. Schubert (1910) included this species in the genus Globigerina, which was later included in Globigerinoides. A new genus Globigerinoidesella was introduced by El-Naggar (1971) to differentiate the forms with radially elongated digitate protuberances on chambers from the other species of Globigerinoides (Spezzaferri et al., 2015), with Globigerina fistulosa Schubert as its type species. Schiebel and Hemleben (2017), following André et al. (2013), identify this species as Globigerinoides sacculifer forma fistulosus, a rarely occurring morphotype in the modern plankton. They opine that a clear distinction between morphotypes forming the fistulose and sac-like chambers may be impossible.
Poole and Wade (2019) refrained from classifying the transitional forms with fistulose protuberances occurring until recent as G. fistulosa, and considered them extreme variants of T. sacculifer to maintain the biostratigraphic utility of G. fistulosa. In Hole U1474A, this species occurs in extremely low abundance and was observed intermittently.
Genus Globoquadrina Finlay 1947
Type species Globorotalia dehiscens Chapman, Parr and Collins, 1934
Globoquadrina dehiscens (Chapman, Parr and Collins 1934)
(Plate P7, figures 1–6)
Basionym: Globorotalia dehiscens
Synonym: Globoquadrina dehiscens
Type species: Globoquadrina dehiscens Chapman, Parr and Collins, 1934
References: Chapman et al. (1934), Bolli et al. (1957), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Fox and Wade (2013), Wade et al. (2018), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 23H-7, 54–56 cm
Remarks. G. dehiscens is an important biostratigraphic marker in both tropical and temperate waters (Jenkins, 1971; Srinivasan and Kennett, 1981b). It has a compact test with a flat spiral and strongly convex umbilical side. There are three to four compressed chambers with a triangular outline and wide umbilicus surrounded by the steep walls of the chambers (Wade et al., 2018), which are also referred to as umbilical shoulders (Kennett and Srinivasan, 1983). The surface is cancellate with circular pores and polygonal ridges. The umbilicus is deep and has apertural tooth.
This cosmopolitan species (Wade et al., 2018) is considered an intermediate dweller by Keller (1985). G. dehiscens is extremely rare in Hole U1474A and is present only in the samples spanning the Late Miocene.
Genus Globorotaloides Bolli, 1957
Type species Globorotaloides variabilis Bolli, 1957
Globorotaloides hexagonus (Natland 1938)
(Plate P7, figures 7–12)
Basionym: Globigerina hexagona
Synonyms: Globorotaloides hexagona, Globoquadrina hexagona, Globorotalia extans Jenkins (1960), Globorotaloides permicrus Blow and Banner (1962), Globigerina clippertonensis McCulloch (1977).
Type species: Globorotaloides hexagonus Natland, 1938
References: Natland (1938), Parker (1962), Lipps (1964), Kennett (1973), Keller (1978), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. hexagonus is an extant form typified by a low trochospiral test with flat spiral side, four to five chambers in the final whorl, and a typical cancellate surface (Kennett and Srinivasan, 1983; Schiebel and Hemleben, 2017) showing honeycomb-like structure (Coxall and Spezzaferri, 2018). It typically consists of five chambers in the final whorl (Kennett and Srinivasan, 1983; Lam and Leckie, 2020a), but in the present study the four-chambered forms were dominant and five-chambered forms were rare. The surface is coarsely cancellate with T. sacculifer-type wall, with pores in hexagonal pore pits (Kennett and Srinivasan, 1983).
G. hexagonus is an extremely rare form restricted to the Indian and Pacific Oceans (Bé and Tolderlund, 1971). It is a deep subthermocline dweller (Ortiz et al., 1996; Birch et al., 2013), commonly occurring in the tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). In Hole U1474A, G. hexagonus is extremely rare in occurrence.
Genus Globoturborotalita Hofker 1976
Type species Globigerina rubescens Hofker 1956
Globoturborotalita apertura (Cushman 1918)
(Plate P7, figures 13–20)
Basionym: Globigerina apertura
Synonym: Globigerina (Zeaglobigerina) apertura
Type species: Globoturborotalita apertura Cushman, 1918
References: Cushman (1918), Kennett and Vella (1975), Kennett and Srinivasan (1983), Iaccarino (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 8H-3, 3–5 cm
Remarks. G. apertura is characterized by a large, low trochospiral test with quadrilobate outline, and a large semicircular aperture with a distinct rim. It has a cancellate surface ultrastructure resembling G. woodi. Blow (1969) considered G. bulloides as the ancestor of G. apertura, but the lack of G. bulloides-type wall and rimmed aperture phylogenetically links it with G. woodi (Kennett and Srinivasan, 1983).
G. apertura preferred open ocean mixed-layer habitat (Aze et al., 2011) and ranged from subtropical to temperate latitudes (Kennett and Srinivasan, 1983). In the present study, it is a rarely occurring species.
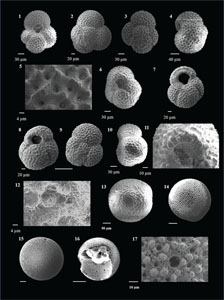
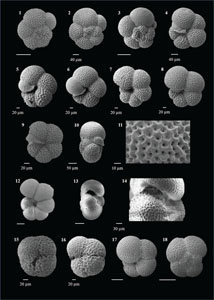
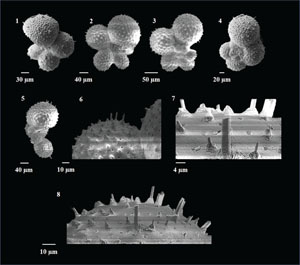
Globoturborotalita decoraperta (Takayanagi and Saito 1962)
(Plate P8, figures 1–4)
Plate P8. Globoturborotalita decorpaerta, Globoturborotalita druryi, Globoturborotalita nepenthes, and Globoturborotalita rubescens.
Basionym: Globigerina druryi decoraperta
Synonym: Globigerina (Zeaglobigerina) decoraperta
Type species: Globoturborotalita decoraperta (Takayanagi and Saito, 1962)
References: Takayanagi and Saito (1962), Kennett (1973), Kennett and Vella (1975), Kennett and Srinivasan (1983), Iaccarino (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 7H-3, 131–133 cm
Remarks. G. decoraperta closely resembles G. woodi but for its high-spired test, which also distinguishes it from G. apertura because both have a large aperture bordered by a rim. G. decoraperta has a compact test with cancellate surface and wide and deep umbilicus with a large, rimmed aperture. This tropical–subtropical species is a mixed-layer dweller (Kennett and Srinivasan, 1983; Aze et al., 2011).
It is an important biostratigraphic marker but has a quite variable recorded stratigraphic range. The LO of G. decoraperta has been assigned a Late Pliocene age by Kennett and Srinivasan (1983), Kaushik et al. (2020), and Lam and Leckie (2020b). Jenkins and Srinivasan (1986), Sinha and Singh (2008), and Singh and Sinha (2022) have reported the LO as Quaternary. The LO of G. decoraperta was observed during the late Early Quaternary in the present study. It is a prominently occurring species in the present work, with a higher relative abundance during the Late Miocene and Pliocene than the Pleistocene in Hole U1474A.
Globoturborotalita druryi (Akers 1955)
(Plate P8, figures 5–8)
Synonym: Globigerina (Zeaglobigerina) druryi
Type species: Globoturborotalita druryi Akers, 1955
References: Akers (1955), Kennett (1973), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 18H-4, 133–135 cm
Remarks. G. druryi is characterized by a small, compact test with lobate periphery and coarsely pitted surface. It has a distinct low arch aperture with a rim, which distinguishes it from G. woodi (Kennett and Srinivasan, 1983; Lam and Leckie, 2020a).
G. druryi was a mixed-layer dweller (Aze et al., 2011) and inhabited lower latitudes (Kennett and Srinivasan, 1983).
In the present study, it is a commonly occurring species in the lower part of the core, with high relative abundance (>10%) in the Late Miocene–Early Pliocene part of the core.
Globoturborotalita nepenthes (Todd 1957)
(Plate P8, figures 9–16)
Basionym: Globigerina nepenthes
Synonym: Globigerina (Zeaglobigerina) nepenthes
Type species: Globoturborotalita nepenthes Todd, 1957
References: Todd (1957), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Hornibrook et al. (1989), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 18H-1, 39–41 cm
Remarks. G. nepenthes shows a compactly coiled test with a characteristic thumb-like protruding final chamber, cancellate wall, and broad aperture with distinct rim (Kennett and Srinivasan, 1983). Its final chamber resembles the tropical insectivorous pitcher plant Nepenthes, from which it derives its name. G. nepenthes differs from its ancestor G. druryi in its final chamber and relatively large test. The wide range of variations in G. nepenthes led to the erection of some other species like Globigerina picassiana (Perconig, 1968) and Globigerina nepenthes delicatula (Brönnimann and Resig, 1971), which were later considered phenotypic variants of G. nepenthes by Kennett and Srinivasan (1983).
This mixed-layer dweller (Aze et al., 2011) lived in warm low latitudes (Kennett and Srinivasan, 1983). G. nepenthes is a frequently occurring species in the lower part of the core and has an average relative abundance of 3%–5%.
Globoturborotalita rubescens (Hofker 1956)
(Plate P8, figures 17–20; Plate P9, figures 1–5)
Plate P9. Globoturborotalita rubescens, Globoturborotalita woodi, Orbulina suturalis, and Orbulina universa.
Basionym: Globigerina rubescens
Synonyms: Globigerina (Zeaglobigerina) rubescens, Globigerina rosacea Bermúdez and Seiglie (1963)
Type species: Globoturborotalita rubescens Hofker (1956)
References: Hofker (1956), Kennett and Srinivasan (1983), Iaccarino (1985), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-18H-7, 35–37 cm, to 1H-1, 0–2 cm
Remarks. G. rubescens is the only extant species of genus Globorotalita (Brummer and Kučera, 2022). It evolved from G. decoraperta and is distinguished by its small size, relatively delicate test, and small circular aperture. The aperture is bordered by a rim. The surface ultrastructure is cancellate, as well as spinose (Aze et al., 2011). G. rubescens often exhibits pink-pigmented tests, especially in modern waters and Late Quaternary sediments.
G. rubescens prefers open ocean mixed-layer habitat (Aze et al., 2011) and is a tropical to subtropical dweller (Kennett and Srinivasan, 1983). Parker (1962) and Hemleben et al. (1989) suggested that white tests of G. rubescens are frequently found in bottom sediments underlying temperate waters and suggested that this species is ubiquitous in tropical to temperate surface waters.
In the present work, the relative abundance of this species is very significant and often exceeds 15% of the total faunal composition.
Globoturborotalita woodi (Jenkins 1960)
(Plate P9, figures 6–12)
Synonym: Globigerina (Zeaglobigerina) woodi
Type species: Globoturborotalita woodi Jenkins, 1960
References: Jenkins (1960), Kennett and Srinivasan (1983), Jenkins (1985), Chaproniere (1988), Spezzaferri (1994), Li and McGowran (2000), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 6H-3, 34–36 cm
Remarks. G. woodi is distinguished by its quadrilobate outline, with a centrally placed, high-arched symmetrically rounded aperture bordered by a distinct rim. It has a cancellate surface ultrastructure with pores located in subhexagonal pore pits.
Pearson et al. (1997), based on isotope studies, assigned surface mixed-layer habitat for G. woodi, whereas Keller (1985) suggested a deeper habitat for this species. G. woodi had a wide latitudinal range from temperate to warm subtropical (Kennett and Srinivasan, 1983). It was a commonly occurring species at mid latitudes in both hemispheres (Lam and Leckie, 2020a; present study). In the present work, G. woodi was a rare species, showing sporadically high abundance in the lower part of the core spanning the Pliocene.
Type species Orbulina universa d'Orbigny, 1839
Orbulina suturalis (Brönnimann 1951)
(Plate P9, figures 13–14)
Synonym: Candorbulina universa Jedlitschka (1934)
Type species: Orbulina suturalis Brönnimann (1951)
References: Brönnimann, (1951), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Norris 1998), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 3H-1, 41–43 cm
Remarks. O. suturalis is a distinct species with a spherical test, areal apertures, and supplementary apertures located along a suture line that joins the previous chambers of the Globigerina stage with the final chamber. The surface ultrastructure is hispid. The large final chamber does not completely envelop the previous chambers, which are visible as low, round projections. The sutural supplementary apertures may sometimes become obscured due to heavy encrustation or secondary infilling, which makes it difficult to identify because the specimen looks like O. universa. Therefore, it sometimes becomes important to locate the projections of the chambers of the Globigerina stage by using a dark-colored dye dripped on the specimen to identify this species.
It has a wide latitudinal range, from tropics to temperate region (Kennett and Srinivasan, 1983). It is widely regarded as a mixed-layer dweller, but Aze et al. (2011) classify it as an open ocean thermocline dweller based on its heavy δ18O signatures.
This species shows sporadic occurrence in Hole U1474A, with abundance rarely exceeding 1% during the Quaternary and Late Pliocene, whereas the Early Pliocene witnessed quite high abundance, occasionally exceeding 15% of the total population.
Orbulina universa (d'Orbigny 1839)
(Plate P9, figures 15–17; Plate P10, figures 1–2)
Type species: Orbulina universa d'Orbigny, 1839
Variant: Orbulina bilobata d'Orbigny (Biorbulina bilobata)
References: d'Orbigny (1839), d'Orbigny (1846), Blow (1956), Postuma (1971), Stainforth et al. (1975), Saito et al. (1981), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Loeblich and Tappan (1994), de Vargas et al. (1999), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. O. universa, a commonly encountered species inhabiting the surface waters of the world oceans from the Subarctic to Subantarctic latitudes (de Vargas et al., 1999), is easily distinguishable by its spherical test formed at the terminal ontogenetic stage (Schiebel and Hemleben, 2017). The development of a completely spherical form in O. universa is believed to be the result of rapid anagenesis at the early/middle Miocene boundary, followed by morphological stasis (Jenkins, 1968; de Vargas et al., 1999). It consists of a single spherical chamber, which is the final chamber enveloping the early part of the test that represents the preadult Globigerina stage (Schiebel and Hemleben, 2017). The surface has hispid wall (Aze et al., 2011) and is densely perforate with two distinct pore sizes, of which the larger ones act as the aperture. The size and frequency of pores in O. universa have been used in paleotemperature studies and it has been observed in laboratory experiments that increases in temperature correlate with larger pore diameter (Caron et al., 1987; Bijma et al., 1990). The tests with large diameter and higher porosities occur in tropical latitudes, whereas those with smaller test diameter and lower porosities are characteristic of mid to high latitudes (Bé and Tolderlund, 1971). Molecular genetic data have revealed three cryptic species of O. universa whose distribution is related to hydrographic provinces and sea-surface total chlorophyll-a concentration (de Vargas et al., 1999). The three cryptic species are regionally separated by their dominance as Caribbean species (Type I), Mediterranean species (Type II), and Sargasso species (Type III), with varying pore density and pore/aperture sizes (de Vargas et al., 1999; Morard et al., 2009; Schiebel and Hemleben, 2017).
O. universa has largely been considered a mixed-layer dweller, although Aze et al. (2011) marked it as an open ocean thermocline species based on its stable oxygen isotope signatures.
Another variant, O. bilobata, which was previously considered an extant species by Kennett and Srinivasan (1983), has been disregarded as a separate species and included in O. universa (Stainforth et al., 1975; Saito et al., 1981; Rossignol et al., 2011; Schiebel and Hemleben, 2017; Brummer and Kučera, 2022). It is an aberrant form of O. universa that develops a second terminal chamber in response to higher food availability (Robbins, 1988; Hemleben et al., 1989). The relative abundance of O. universa is higher during the Pliocene as compared to Quaternary, occasionally exceeding 10% during the Pliocene.
Genus Sphaeroidinella Cushman 1927
Type Species Sphaeoridina bulloides d'Orbigny var. dehiscens Parker and Jones, 1865
Sphaeroidinella dehiscens (Parker and Jones 1865)
(Plate P10, figures 3–11)
Basionym: Sphaeroidina bulloides var. dehiscens
Synonyms: Sphaeroidinella dehiscens immatura Cushman (1919), Sphaeroidinella dehiscens excavata Banner and Blow (1965), Sphaeroidinella dehiscens ionica ionica Cita and Ciaranfi (1972), Sphaeroidinella ionica evoluta Cita and Ciaranfi (1972)
Type species: Sphaeroidinella dehiscens Parker and Jones, 1865
References: d'Orbigny (1865), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Pearson (1995), Malmgren et al. (1996), Kučera (1998), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-16H-4, 67–69 cm, to 1H-1, 0–2 cm
Remarks. S. dehiscens is an extant species characterized by a large, ovoid test with three chambers in the final whorl. It has a deep umbilicus, a large primary aperture, and a secondary sutural aperture. The apertures are bordered by crenulated lip. It has a nonspinose cancellate wall with cortex (Aze et al., 2011). S. dehiscens differs from S. paenedehiscens in having secondary apertures (Kennett and Srinivasan, 1983).
The development of a secondary aperture in the test of S. dehiscens is considered an important event in Late Neogene biostratigraphy. Berggren et al. (1985) assigned an age of 5.1 Ma to the evolutionary appearance of secondary apertures, which was revised by Malmgren et al. (1996) to 5.5 Ma. The evolution of S. dehiscens from S. paenedehiscens by development of minute secondary apertures in the latest Miocene (Malmgren et al., 1996; Kučera, 1998) was used to define the base of Zone N19 (Kennett and Srinivasan, 1983). These forms with minute secondary apertures were referred to as S. dehiscens forma immatura (Kučera, 1998). Such forms were also encountered in the lower part of the studied core in the present study (Plate P25, figures 17–19). The transition from such minute secondary aperture to a large secondary aperture covering the spiral side occurred after the mid Pliocene (Malmgren et al., 1996) and marked the appearance of S. dehiscens sensu stricto (Bandy, 1964).
Sphaeroidinella is a monospecific genus that develops the cortex at the terminal stage of its life cycle, which makes the preadult specimens similar to T. sacculifer (Schiebel and Hemleben, 2017; Brummer and Kučera, 2022).
S. dehiscens is an open ocean thermocline dweller (Aze et al., 2011), ranging from tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). It occurs in extremely low abundance in Hole U1474A.
Genus Sphaeroidinellopsis Banner and Blow 1959
Type species Globigerina seminulina Schwager 1866
Sphaeroidinellopsis kochi (Caudri 1934)
(Plate P10, figures 12–19; Plate P11, figures 1–5)
Plate P11. Sphaeroidinellopsis kochi, Sphaeroidinellopsis paenedehiscens, and Sphaeroidinellopsis seminulina.
Synonym: Sphaeroidinella rutschi Cushman and Renz (1941), Sphaeroidinella multiloba LeRoy (1944), Globigerina grimsdalei Keijzer (1945), Sphaeroidinellopsis hancocki Bandy (1975).
Type species: Sphaeroidinellopsis kochi Caudri, 1934
References: Caudri (1934), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Pearson (1995), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 14H-3, 21–23 cm
Remarks. S. kochi differs from Sphaeroidinellopsis seminulina in having more than three chambers (Kennett and Srinivasa, 1983), a wide open umbilicus, and a larger aperture. It has four to five chambers in the final whorl, with last chamber of characteristic sacculiferid shape (Bolli and Saunders, 1985). The wall is distinctly cancellate in the specimens that lack a cortex, whereas those with cortex exhibit smooth surface. The aperture has crenulated margin like S. seminulina. In some specimens of S. kochi, series of supplementary sutural apertures were visible on the spiral side, but these appear to be more of a diagenetic signature rather than a distinguishing morphological feature.
S. kochi was a thermocline dweller (Aze et al., 2011) found in tropical latitudes (Kennett and Srinivasan, 1983). In Hole U1474A, it is found in low abundance.
Sphaeroidinellopsis paenedehiscens (Blow 1969)
(Plate P11, figures 6–8)
Basionym: Sphaeroidinella subdehiscens paenedehiscens
Synonym: Sphaeroidinellopsis sphaeroides Lamb (1969).
Type species: Sphaeroidinellopsis paenedehiscens Blow, 1969
References: Blow (1969), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 15H-4, 67–69 cm
Remarks. S. paenedehiscens has a large test with rounded periphery, with three inflated chambers in the final whorl, and a heavy cortex that obscures the pores and sutures. The wall is cancellate with smooth cortex (Aze et al., 2011). The aperture is elongate and has a crenulated lip. S. paenedehiscens differs from S. seminulina in having a large test, more spherical outline, and a more elongated aperture. Another important distinguishing feature is the compactness of the test that gives the impression of two chambers in the final whorl, unlike S. seminulina, in which the three chambers are distinct.
S. paenedehiscens was an open ocean thermocline dweller (Aze et al., 2011) found in tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). In Hole U1474A, S. paenedehiscens occurs in extremely low abundance in the Pliocene samples.
Sphaeroidinellopsis seminulina (Schwager 1866)
(Plate P11, figures 9–18)
Basionym: Globigerina seminulina
Synonym: Sphaeroidinellopsis seminulina seminulina, Sphaeroidinella spinulosa Subbotina (1958), Sphaeroidinellopsis subdehiscens Blow (1959).
Type species: Sphaeroidinellopsis seminulina Schwager, 1866
References: Schwager (1866), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 12H-1, 71–73 cm
Remarks. S. seminulina is characterized by a compact test with a broadly ovate to trilobulate outline and is covered with a heavy cortex (Kennett and Srinivasan, 1983). It has three chambers in the final whorl, a cancellate wall that shows smooth texture due to the cortex, and an elongate umbilical aperture with crenulated margin. The coarsely perforate surface has cancellate wall, which is covered by a heavy cortex giving it a glossy appearance (Kennett and Srinivasan, 1983). In almost all the specimens encountered, the surface of the last chamber of the final whorl showed blunt, spine-like extensions of the cortex.
A lot has been discussed about the number of chambers in the final whorl of S. seminulina, and several species/subspecies have been proposed. The original description of S. seminulina by Schwager (1866) indicates three-chambered form, which was different from the four-chambered neotype selected by Banner and Blow (1960). Another species, S. subdehiscens, was erected by Blow (1959) for three-chambered forms. Later, Srinivasan and Kennett (1981b), on comparison of both the forms, concluded that S. subdehiscens was a junior synonym of S. seminulina. Lam and Leckie (2020a) included the forms with three to three and a half chambers in S. seminulina. In the present work, we have adhered to the concept of Kennett and Srinivasan (1983) and included only three-chambered forms in S. seminulina. The forms with more than three chambers in the final whorl were included in S. kochi.
S. seminulina was a thermocline dweller (Aze et al., 2011) extending in the tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). In Hole U1474A, S. seminulina showed extremely high abundance during the Late Miocene–Early Pliocene, often exceeding 10% of the faunal population, whereas during the Late Pliocene it showed extremely low abundance.
Genus Trilobatus Spezzaferri et al. 2015
Type species Trilobatus trilobus
Trilobatus quadrilobatus (d'Orbigny 1846)
(Plate P12, figures 1–4)
Basionym: Globigerina quadrilobata
Synonym: Globigerinoides quadrilobatus, Globigerinoides primordius
Type species: Trilobatus quadrilobatus d'Orbigny (1846)
References: d'Orbigny (1846), Banner and Blow (1960), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Spezzaferri et al. (2018), Poole and Wade (2019)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. T. quadrilobatus is characterized by three and a half to four chambers, a T. sacculifer-type wall, and a low arch primary aperture bordered by a thin rim centered over the antepenultimate chamber (Schiebel and Hemleben, 2017). There are two supplementary apertures on the spiral side.
This species closely resembles Trilobatus immaturus but for its higher aperture (Kennett and Srinivasan, 1983). In the present work, both species are clubbed into T. quadrilobatus. André et al. (2013) included it in T. sacculifer based on molecular genetic studies.
T. quadrilobatus is a cosmopolitan species (Kennett and Srinivasan, 1983; Spezzaferri et al., 2018) and prefers mixed-layer habitat (Chaisson and Ravelo, 1997). It is a consistently occurring species with low abundance in Hole U1474A.
Trilobatus sacculifer (Brady 1877)
(Plate P12, figures 5–14)
Basionym: Globigerina sacculifera
Synonyms: Globigerinoides sacculifer, Globigerina bulloides var. recumbens Rhumbler (1901)
Type species: Trilobatus sacculifera Brady (1877)
References: Brady (1877), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Spezzaferri et al. (2018), Poole and Wade (2019), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. T. sacculifer is an important mixed-layer dwelling species, abundant in tropical–subtropical latitudes (Kennett and Srinivasan, 1983; Schmuker and Schiebel, 2002; Aze et al., 2011; Schiebel and Hemleben, 2017). It has a unique morphological appearance and can be distinguished by its compressed, sac-like final chamber, which shows several modifications. The test has a T. sacculifer-type wall, a high arch, and rimmed primary aperture. There are numerous supplementary apertures on the spiral side.
T. sacculifer has been studied in great detail by several authors for its micropaleontological as well as molecular genetic aspects (e.g., Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; André et al., 2013; Spezzaferri et al., 2015, 2018; Schiebel and Hemleben, 2017; Poole and Wade, 2019; Lam and Leckie, 2020a). It was considered a descendant of the lineage triloba-immaturus-quadrilobatus-sacculifer by Kennett and Srinivasan (1983) and included in the Group B stock of their classification of Globigerinoides. As previously discussed, André et al. (2013) grouped triloba, immaturus, quadrilobatus, and sacculifer as the morphospecies of T. sacculifer. Lam and Leckie (2020a) have preferred the biological definition of this species and grouped T. triloba with T. sacculifer due to their similar stratigraphic ranges in the northwest Pacific Ocean. Schiebel and Hemleben (2017) opine that of the various morphotypes, sacculifer is the valid species name because it best includes the entire range of the morphotypes and have assigned terminology like forma trilobus, forma quadrilobatus and forma sacculifer to express their concept of genotype. Poole and Wade (2019) encountered several forms of T. sacculifer with an elongate fistulose projection from the final chamber and considered it to be the plexus form of T. sacculifer.
Although in modern plankton, all these morphotypes are considered conspecific (André et al., 2013), in the fossil record they are still regarded as distinct species due to their different stratigraphic ranges (Spezzaferri et al., 2018). The census data suggest low abundance of this commonly occurring species in Hole U1474A.
Trilobatus trilobus (Reuss 1850)
(Plate P12, figures 15–18)
Synonym: Globigerinoides triloba, Globigerinoides trilobus Spezzaferri (1994)
Type species: Trilobatus triloba Reuss (1850)
References: Reuss (1850), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Spezzaferri (1994), Fox and Wade (2013), Spezzaferri et al. (2015), Schiebel and Hemleben (2017), Poole and Wade (2019)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. T. trilobus is characterized by its low trochospiral test with three chambers in the last whorl, with the final chamber being larger than the previous two. The surface ultrastructure is cancellate with T. sacculifer-type wall. The primary aperture is a narrow slit-like, sometimes low arch, and there are two to three secondary apertures on the spiral side.
There has been a large debate on the status of T. trilobus as a separate species. Earlier, this species was considered under the genus Globigerinoides, which was later included in the genus Trilobatus (Spezzaferri et al., 2015). Spezzaferri et al. (2015), using stratophenetic and molecular genetic data, proved the polyphyletic origin of the genus Globigerinoides, a trait previously discussed by several authors, for example, Takayanagi and Saito (1962), Keller (1981), Kennett and Srinivasan (1983), Jenkins (1985), Spezzaferri and Premoli Silva (1991), and Spezzaferri (1994). André et al. (2013) conducted molecular genetic studies on modern forms and concluded that the species trilobus, immaturus, quadrilobatus, and sacculifer are the morphotypes of the same species, which are not separated by the biologists and included in T. sacculifer. However, the fossils of these forms are still considered separate species and used exclusively for paleoceanographic and biostratigraphic studies (Spezzaferri et al., 2018; Poole and Wade, 2019; Lam and Leckie, 2020a, 2020b). Schiebel and Hemleben (2017) described four common morphotypes of the modern T. sacculifer (Globigerinoides sacculifer, as mentioned by the authors): forma trilobus, forma quadrilobatus, forma immaturus, and forma sacculifer. All these forms are considered morphotypes of T. sacculifer due to its range that includes the entire range of morphotypes including the plexus forms (Schiebel and Hemleben, 2017). The focus of the present work is on the paleoceanographic reconstruction of the Agulhas Current, so these forms are considered as separate distinct species.
T. trilobus is very abundant in the tropics as well as commonly found in the mid latitudes (Spezzaferri et al., 2018) and prefers mixed-layer habitat (Pearson et al., 1997). In the present work, this species is found consistently, with an abundance of 2%–3%.
Genus Turborotalita Blow and Banner, 1962
Type Species Truncatulina humilis Brady 1884
Turborotalita quinqueloba (Natland 1938)
(Plate P13, figures 1–7)
Basionym: Globigerina quinqueloba
Synonym: Globigerina quinqueloba
Type species: Turborotalita quinqueloba Natland, 1938
References: Natland (1938), Kennett and Srinivasan (1983), Jenkins (1985), Pearson and Wade (2009), Schiebel and Hemleben (2017), Pearson and Kučera (2018), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. T. quinqueloba shows wide morphological variability and resembles many forms with ampullate final chambers (Brummer and Kučera, 2022). The test consists of four and a half to five chambers in the final whorl, with the final chamber modified in a flap-like extension, which resembles a lip in some morphotypes and is modified like a bulla in others. The surface ultrastructure consists of a primarily smooth wall with the development of G. ruber/T. sacculifer-type wall, also known as Turborotalita-type wall ultrastructure (Hemleben and Olsson, 2006). Another important feature is the distinctly spinose surface of the last chamber, with short, stunted triangular spines. It is a species inhabiting subpolar latitudes (Bé and Tolderlund, 1971). T. quinqueloba is rare in Hole U1474A and occurs sporadically, with the relative abundance below 0.5%.
Turborotalita humilis (Brady 1884)
(Plate P13, figure 8)
Basionym: Truncatulina humilis
Synonym: Turborotalita humilis, Turborotalita cristata
Type species: Turborotalita humilis Brady, 1884
References: Brady (1971), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Brummer and Kučera (2022)
Remarks. T. humilis is an extant form (Schiebel and Hemleben, 2017), characterized by a small, compact test with circular equatorial outline and six chambers in the final whorl. The last chamber is characteristically modified in the form of tongue-like extension over the umbilicus and has several infralaminal apertures (Kennett and Srinivasan, 1983). The extension of the last chamber also resembles bulla that wraps the umbilicus and ends in a series of finger-like extensions over the umbilical sutures (Bolli and Saunders, 1985). The wall is spinose and shows cancellate structure (Aze et al., 2011), which is often obscured due to encrusting.
T. humilis is a mixed-layer dweller found in tropical–subtropical latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011). It is extremely rare in Hole U1474A and occurred sporadically.
Family GLOBIGERINITIDAE Bermúdez 1961, revised Li 1987; Pearson and Wade 2009
Genus Globigerinita Brönnimann 1951
Type species Globigerinita naparimaensis Brönnimann 1951
Globigerinita glutinata (Egger 1893)
(Plate P13, figures 9–19)
Basionym: Globigerina glutinata
Synonyms: Globigerinita glutinata glutinata, Globigerinita naparimaensis Brönnimann (1951), Globigerinita incrusta Akers (1955), Globigerinita parkerae Bermúdez (1961), Globigerinita flparkerae Brönnimann and Resig (1971)
Type species: Globigerinita glutinata Egger, 1893
References: Egger (1893), Parker (1962), Lipps (1964), Fleisher (1974), Keller (1978), Kennett and Srinivasan (1983), Chaisson and Leckie (1993), Spezzaferri (1994), Pearson (1995), Schiebel and Hemleben (2017), Pearson, Wade and Huber (2018), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. glutinata is a ubiquitous species in the modern ocean, most abundant in subtropical to temperate waters (Schiebel and Hemleben, 2017). It is an important upwelling indicator in the lower latitudes (Sinha et al., 2006).
G. glutinata is characterized by a small compact test, with four chambers in the last whorl. The surface is smooth, with irregularly distributed fine perforations covered by crystallites. It has umbilical aperture with a characteristic inflated bulla that covers the umbilicus. The inflation of bulla along the umbilical sutures shows numerous infralaminal supplementary apertures bordered by tubulose openings (Kennett and Srinivasan, 1983).
There is considerable morphological variability in the development of bulla in G. glutinata and its genetic diversity (André et al., 2014; Schiebel and Hemleben, 2017; Morard et al., 2019; Brummer and Kučera, 2022).
Another morphotype, Globigerinita parkerae Bermúdez (1961), which was previously considered a distinct species (Kennett and Srinivasan, 1983), is now a synonym of G. glutinata (Brummer and Kučera, 2022). G. parkerae differs from G. glutinata by secondary apertures on the spiral side. It was considered the ancestor of Candeina nitida (Kennett and Srinivasan, 1983). Following Brummer and Kučera (2022), it is lumped with G. glutinata in the present work.
It is an important species in paleoceanographic reconstructions, as it is used as an upwelling indicator. G. glutinata is a shallow mixed-layer dweller (Pearson and Wade, 2009) and shows a global occurrence (Pearson et al., 2018).
In Hole U1474A, G. glutinata is a prominent species that occurs in high abundance. It is present throughout the core and has higher abundance during Quaternary than Pliocene.
Family GLOBOROTALIIDAE Blow 1979
Type species Globorotalia conomiozea Kennett 1966
Globoconella conomiozea (Kennett 1966)
(Plate P14, figures 1–10)
Basionym: Globorotalia conomiozea
Type species: Globoconella conomiozea Kennett (1966)
References: Kennett (1966, 1973), Chaproniere (1973), Kennett and Vella (1975), Hornibrook (1982), Kennett and Srinivasan (1983), Jenkins (1985), Jenkins and Srinivasan (1986), Cifelli and Scott (1986), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-24H-4, 56–58 cm, to 17H-5, 107–109 cm
Remarks. G. conomiozea is characterized by a thick, planoconvex test, with a highly vaulted umbilical side, strong marginal keel, and high arched aperture. A lot of confusion has prevailed over the differences between G. miotumida and G. conomiozea because of the subtle variation in the vaulted umbilical side. Kennett (1966) described this species as having a planoconvex test with a flat spiral side and a strongly vaulted umbilical side. This characteristic is also exhibited by G. miotumida tests lacking secondary calcification, which can show a strongly pronounced keel and vaulted umbilical side (Lam and Leckie, 2020a). It makes G. miotumida gradational with G. conomiozea. Lam and Leckie (2020a) attempted to differentiate the two species by projecting a line from either side of the test on the axial view. They have assigned the forms in which the angle made between the lines is less than 90° to G. conomiozea suggesting the umbilical side is more highly vaulted than G. miotumida in which the angle was equal to or greater than 90°. This approach is quite cumbersome when it comes to working with a large number of samples, using a light microscope, like in the present study. Also, if the G. miotumida tests are encrusted with secondary calcite, it would for sure show an angle close to G. conomiozea.
In the present study, differentiation between the two species is done taking into account the test thickness and aperture height. The test of G. conomiozea is thicker and more vaulted on the umbilical side, resulting in an elongate and relatively lower aperture than G. miotumida, which has a less thick test, less vaulted umbilical side, and a higher aperture.
The presence of keel is a distinguishing feature of G. conomiozea among the Globoconella lineage. There was a distinct loss of keel in the course of evolution of this genus during the evolution from G. conomiozea to G. puncticulata. The loss of keel in the Globoconella lineage is an important morphological change that occurred in the Miocene in response to cooling (Malmgren and Kennett, 1981). The keel, almost universally present in the globorotalid taxa with compressed chambers, is like a crimp in a wall that acts a support to the periphery, and is not present in forms with inflated chambers (Scott et al., 1990). There was a rapid loss of keel in the forms that descended from G. conomiozea (Malmgren and Kennett, 1981; Kennett and Srinivasan, 1983; Scott et al., 1990). Walters (1965) erected a new species Globoconella sphericomiozea descending from G. conomiozea, distinguished by small its small, compact test and rapid loss of keel (Kennett and Srinivasan, 1983). Later, Scott et al. (2007) considered G. sphericomiozea as a subspecies of G. puncticulata, whereas Lam and Leckie (2020a) synonymized it with G. puncticulata.
In the present work, the forms with even a remnant of keel are included in G. conomiozea, thereby leading to nonrecognition of G. sphericomiozea as a separate species and synonymizing it with G. puncticulata. A detailed discussion is presented in the remarks section of G. puncticulata.
G. conomiozea is a temperate species (Kennett and Srinivasan, 1983) and prefers thermocline habitat (Aze et al., 2011). It is an important indicator of events of incursion of the cold Southern Ocean water to Hole U1474A in the present study. It showed an average abundance of 1%–3% in the samples spanning Early Pliocene, occasionally exceeding 7% of the total assemblage.
Globoconella inflata (d'Orbigny 1839)
(Plate P14, figures 11–20; Plate P15, figures 1–7)
Type species: Globoconella inflata d'Orbigny (1839)
References: d'Orbigny (1839), Jenkins (1971), Kennett (1973), Kennett and Vella (1975), Maiya et al. (1976), Keller (1978), Kennett and Srinivasan (1983), Iaccarino (1985), Hornibrook et al. (1989), Scott et al. (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-16H-5, 77–79 cm, to 1H-1, 0–2 cm
Remarks. G. inflata is an extant species (Brummer and Kučera, 2022), characterized by its globose test, with three to three and a half inflated chambers in the final whorl, high arch aperture, and a pustulose surface, often covered with a cortex giving it a porcelaneous texture. The cortex is formed of interlocking calcitic plates, sometimes with irregular pores distributed on it. Although Aze et al. (2011) have marked a cancellate surface of the wall, it was not observed in the specimens in the present work. Rather, the wall structure beneath the cortex is porous with ridges in a crisscross manner, giving it a mesh-like appearance (refer to Plate P15, figure 7). The highly globose and tight forms sometimes resemble Pulleniatina obliquiloculata, from which it is distinguished by having a low trochospiral coiling, unlike the latter, which has streptospiral coiling.
There have been several views about the number of chambers in the final whorl of G. inflata. Kennett and Srinivasan (1983) mentioned the presence of three to three and a half chambers, whereas Scott et al. (1990), Schiebel and Hemleben (2017), and Lam and Leckie (2020a) mention the presence of three to four chambers. Kennett and Vella (1975), Malmgren and Kennett (1981), and Kennett and Srinivasan (1983) have categorically mentioned that G. inflata evolved from G. puncticulata by a reduction in the number of chambers and increase in the degree of inflation of the chambers. This warrants unanimity over the number of chambers in the final whorl of G. inflata. In the present work, the name G. inflata has been strictly applied to the forms with three to three and a half chambers only. All the forms with more than three and a half chambers were excluded from G. inflata and were identified on the basis of other associated morphological characteristics.
A few species were identified over the past several decades: Neoacarinina blowi Thompson 1973, Globigerina nipponica Asano 1957, and Globorotalia oscitans Todd 1957, which were later rejected by Brummer and Kučera (2022) as variants of G. inflata. Another species, Globoconella triangula Theyer 1973, is a very closely resembling form with G. inflata. Kennett and Srinivasan (1983) and Chaisson and Pearson (1997) considered it as a variant of G. inflata, whereas Scott et al. (1990) described it as a form normally intergrading with G. inflata yet being distinct in some collections. Lam and Leckie (2020a) consider it a separate species on the basis of a distinct triangular outline in the axial view given by highly vaulted umbilical chambers. In the present work, we refrained from distinguishing G. triangula from G. inflata following Kennett and Srinivasan (1983).
G. inflata prefers subpolar to transitional water mass (Bé, 1977; Hilbrecht, 1996; Sinha et al., 2006; Morard et al., 2011) and has been widely used in tracing the oceanic fronts migration (Peeters et al., 2004; Caley et al., 2014; Morard et al., 2016; Singh et al., 2023; Dwivedi et al., 2024). It has two genetically distinct morphotypes, Type I occurring between the subtropical to subpolar latitudes and Type II occurring mainly southward of subpolar front (Morard et al., 2011, 2013).
G. inflata is a thermocline dweller (Aze et al., 2011) which frequently occurs in the vicinity of hydrologic fronts and eddies (Schiebel and Hemleben, 2017). The increasing abundance of G. inflata at lower latitudes may sometimes indicate selective dissolution of solution-prone species (Bé, 1977; Sinha et al., 2006).
This species has shown significant variation in the present study during the Late Pliocene, with the relative abundance reaching up to 5%, although its variability during the Quaternary was extremely important, with abundance often exceeding 15% during the events of the northward migration of the subtropical front.
Globoconella miotumida (Jenkins 1960)
(Plate P15, figures 8–12)
Synonym: Globorotalia miozea conoidea Walters 1965
Type species: Globoconella miotumida Jenkins (1960)
References: Jenkins (1960, 1985), Walters (1965), Kennett and Vella (1975), Keller (1980), Kennett and Srinivasan (1983), Cifelli and Scott (1986), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 16H-5, 77–79 cm
Remarks. G. miotumida is characterized by a large test with four and a half to five chambers in the final whorl, with a highly conical umbilical side and a cord like peripheral keel. The surface has a nonspinose, finely perforate wall and a distinctly arch aperture. The earlier chambers show secondary thickening on the periphery and umbilical surface, which makes the periphery on the earlier chambers bluntly rounded and thickened, as compared to the later chambers, which are more sharply angled (Kennett and Srinivasan, 1983).
G. miotumida has undergone taxonomic revision following the discussion by several authors. Kennett and Srinivasan (1983) considered Globorotalia conoidea Walters (1965) as a distinct species, and were of the opinion that Globorotalia miotumida may represent a thin-walled form of G. conoidea and should be given a priority. Cifelli and Scott (1986), Scott et al. (1990), and later Lam and Leckie (2020a) synonymized Globorotalia conoidea with Globoconella miotumida. In the present work, G. miotumida has been given priority over G. conoidea following the modern taxonomic concepts. Sometimes the secondary calcification over the test of G. miotumida may obscure its keel and increase the test thickness, making it appear like G. conomiozea.
It is a thermocline dweller, occurring in the temperate to warm subtropical latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011). In the present study it is a rarely occurring form in the Pliocene samples.
Globoconella puncticulata (Deshayes 1832)
(Plate P15, figures 13–17; Plate P16, figures 1–8)
Basionym: Globigerina puncticulata
Synonym: Globorotalia puncticulata
Type species: Globoconella puncticulata Deshayes (1832)
References: Deshayes (1832), Banner and Blow (1960), Kennett (1973), Hornibrook (1982), Kennett and Srinivasan (1983), Jenkins (1985), Jenkins and Srinivasan (1986), Cifelli and Scott (1986), Scott et al. (1990), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-21H-3, 71–73 cm, to 1H-1, 0–2 cm
Remarks. G. puncticulata can be distinguished by its planoconvex test, with a highly vaulted umbilical side and absence of keel. It has four chambers in the final whorl and a high arch aperture with a rim. The periphery is angular–subangular to subrounded. The surface exhibits a smooth, perforate wall, which is densely pustulose on earlier chambers.
The main distinguishing criteria of G. puncticulata from G. conomiozea is the absence of keel and height of the aperture, which is lower in the latter. It was also observed that the tests of G. conomiozea were more robust than G. puncticulata.
The increase in the roundness of the peripheral margin of the test and inflation of the chambers, making them globose, led to the evolution of Globoconella inflata from G. puncticulata. The main features that separate G. inflata from G. puncticulata are the rounded margin, three to three and a half (fewer than four) chambers in the final whorl, and the cortex on the test, giving it a smooth texture.
There have been several discussions about the ancestry and lineage of G. puncticulata with many species. Barbieri (1967) considered it to be closely related to Globorotalia crassaformis, whereas Blow (1969) and later Stainforth et al. (1975) suggested it to have descended from Globorotalia subscitula. Berggren (1977) was of the opinion that it descended from Globorotalia cibaoensis. Later, Kennett (1973) and Malmgren and Kennett (1981) showed that G. puncticulata evolved from G. conomiozea via an intermediate form G. puncticulata sphericomiozea, later giving rise to G. inflata during the Late Pliocene. Jenkins (1985) considered G. puncticulata to have descended from G. sphericomiozea. Kennett (1973) included G. puncticulata in G. sphericomiozea, whereas Walters (1965) considered G. sphericomiozea to have graded into G. inflata, making G. puncticulata an intermediate form between these two species. Thus, large confusion over the ancestry of G. puncticulata has prevailed over several decades. After analyzing all these literatures and the specimens recovered from Hole U1474A, we are of the opinion that G. puncticulata evolved from G. conomiozea by developing more rounded peripheral margin, complete loss of keel, and reduction in the vaulting of the umbilical side.
Walters (1965) erected a new species, G. puncticulata sphericomiozea, which was an intermediate form between G. conomiozea and G. puncticulata. It was distinguished by a small test and rapid loss of keel from its ancestor G. conomiozea (Kennett and Srinivasan, 1983), although a weak keel on the last chamber in many specimens has also been reported (Scott et al., 1990). Another method of distinction was suggested by Scott (1980) based on population approach in which the appearance of G. sphericomiozea would occur if the nonkeeled tests comprised at least 5% of a sample, and G. puncticulata occurred if all the tests were nonkeeled. Although this approach was suggested for biostratigraphy (Scott, 1980), it is our opinion that the population-based approach for taxonomic and biostratigraphic studies does not serve the purpose accurately, as the morphological variations are not dependent on the relative abundance.
Lam and Leckie (2020a) synonymized G. sphericomiozea with G. puncticulata following difficulty in consistent recognition and differentiation of this species due to strong similarities in the test features. The various studies concerning the loss of keel from G. conomiozea and its evolution to G. puncticulata and G. inflata (Kennett, 1966; Kennett and Vella, 1975; Scott, 1979, 1980; Scott et al., 1986, 1990; Kennett and Srinivasan, 1983; Jenkins, 1985) considered it an important event in the course of evolution of this genus, but confusion always loomed whether to include the forms with remnant of keel in G. conomiozea or G. sphericomiozea. Lam and Leckie (2020a) have reported that the original description of Walters (1965), along with the holotype and paratype images are gradational with G. puncticulata and G. conomiozea, and a specimen even had visible keel on the last chamber with more vaulted umbilical chambers. These forms, which had even a part of the keel, were included in G. conomiozea by Lam and Leckie (2020a). In the present work also, this approach is accepted and followed, leading to classifying all the forms with even partial keel as G. conomiozea, whereas only those forms which completely lacked keel were included in G. puncticulata. Other than the absence of keel, some factors like overlapping metrics of globoconellid shape measurements between G. puncticulata and G. sphericomiozea (Malmgren and Kennett, 1981) and the nearly identical signal in the isotopic values in paleoecological study during the Neogene (Schneider and Kennett, 1996), also support synonymizing G. sphericomiozea and G. puncticulata. This resolves the issue of identification of forms with weak/partial keel.
The taxonomic concept pertaining to G. puncticulata s.s. has changed several times over the last few decades. Banner and Blow (1960) comprehensively described this species and assigned a lectotype. Although the definition of this species was accepted by Barbieri (1971), the lectotype was repudiated on the pretext that the lectotype showed fairly high arch aperture, whereas the original description by Deshayes considers it as a small apertura rotunda. This issue of variability in the shape of the aperture from rounded to arched has been dealt with in detail (Stainforth et al., 1975; Jenkins, 1985). The rounded periphery and rounded aperture became the main criteria to classify the forms as G. puncticulata, whereas the forms with rounded periphery and umbilical-extraumbilical elongated apertures were included in Globorotalia crassaformis by Barbieri (1967), which was later supported by Iaccarino (1967), Barbieri and Petrucci (1967), and Barbieri (1971). Jenkins (1985) included all such forms in G. puncticulata, as it showed features consistent with the lectotype description and the variation in the species.
It is a temperate to warm subtropical latitude dweller (Kennett and Srinivasan, 1983) and prefers an open ocean thermocline habitat (Aze et al., 2011).
It is a consistently occurring species in Hole U1474A from Early Pliocene (5.37 Ma) onward, although in lower abundance. It is a surprising observation that this species is constantly found in all the samples of Quaternary to the top of the core.
Genus Globorotalia Cushman 1927
Subgenus Globorotalia Bandy 1972
Type species Pulvinulina menardii var. tumida Brady 1877
Globorotalia (Globorotalia) merotumida (Blow and Banner 1965)
(Plate P16, figures 9–14)
Synonym: Globorotalia merotumida
Type species: Globorotalia merotumida Blow and Banner, 1965
References: Blow and Banner (1965), Keller (1980), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 15H-2, 79–81 cm
Remarks. G. merotumida is characterized by a small biconvex test, with more inflated umbilical side. It has five to six wedge-shaped chambers in the final whorl and a distinct marginal keel. The surface is densely perforate with a smooth wall and limbate sutures. The distinctive feature of G. merotumida is the last chamber of the final whorl, which is as tall as wide (Bolli and Saunders, 1985).
G. merotumida is an open ocean thermocline dweller (Aze et al., 2011) that extends from tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). It is an important biostratigraphic marker in the Late Neogene. In Hole U1474A, it is regularly present in the samples spanning Early to Middle Pliocene but show very low abundance.
Globorotalia (Globorotalia) plesiotumida (Blow and Banner 1965)
(Plate P16, figures 15–18; Plate P17, figures 1–2)
Synonym: Globorotalia plesiotumida
Type species: Globorotalia plesiotumida Blow and Banner, 1965
References: Blow and Banner (1965), Postuma (1971), Kennett and Srinivasan (1983), Cifelli and Scott (1986), King et al. (2020), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 15H-2, 79–81 cm
Remarks. G. plesiotumida differs from G. merotumida by its relatively larger test, an elongate equatorial outline, and the width and height of the last chamber, which is taller than wide in this case. Other features are quite similar to G. merotumida.
G. plesiotumida is an open ocean thermocline dweller (Aze et al., 2011) that extends from tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). It descended from G. merotumida and acts as an important biostratigraphic marker in the Late Neogene. The evolutionary appearance of G. plesiotumida is used to mark the base of the Zone N17, and its evolution to G. tumida tumida approximates Zone N17–N18 (Kennett and Srinivasan, 1983).
In Hole U1474A, it is regularly present in the samples spanning Early to Middle Pliocene but shows very low abundance.
Globorotalia (Globorotalia) tumida (Brady 1877)
(Plate P17, figures 3–11)
Basionym: Pulvinulina menardii var. tumida
Synonym: Globorotalia tumida tumida
Type species: Globorotalia tumida Brady, 1877
References: Brady (1877), Blow (1969), Kennett (1973), Keller (1980), Saito et al. (1981), Hornibrook (1982), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Scott et al. (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-23H-5, 40–42 cm, to 1H-1, 0–2 cm
Remarks. G. tumida has a large, robust biconvex test with a tumid base. The marginal keel is very heavy and cord-like. The surface is densely perforate with pores of uniform size and smooth. The degree of convexity of the test and the thickness of the tumid base shows variation with latitudes, with the tropical forms being tumider than their subtropical counterparts (Chaisson and Leckie, 1993; Lam and Leckie, 2020a).
Often this species has been confused and lumped with G. menardii based on the census counts of the Quaternary assemblages (Brummer and Kučera, 2022). Lam and Leckie (2020a) found that in the Kuroshio Current Extension region, G. tumida showed morphologically similar features to G. menardii. In the present work, the tests of G. tumida were distinct from G. menardii. Another species, G. tumida flexuosa Koch, which was identified as a distinct species by Kennett and Srinivasan (1983) and Bolli and Saunders (1985), has been considered an aberrant form of G. tumida by Brummer and Kučera (2022). The robust and thick test makes G. tumida highly resistant to dissolution, which results in its higher abundance in sediments than the water column (Schiebel and Hemleben, 2017).
G. tumida is an open ocean thermocline dweller (Aze et al., 2011) and occurs in preferably tropical, warm subtropical latitudes (Kennett and Srinivasan, 1983). Berggren (1973) and Fleisher (1974) considered the evolutionary appearance of G. tumida at the base of Zone N18 as an important marker for Miocene/Pliocene boundary.
Globorotalia (Globorotalia) ungulata (Bermúdez 1961)
(Plate P17, figures 12–19)
Synonym: Globorotalia ungulata
Type species: Globorotalia ungulata Bermúdez, 1961
References: Bermúdez (1961), Kennett and Srinivasan (1983), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-14H-5, 109–11 cm, to 1H-1, 0–2 cm
Remarks. G. ungulata is considered to resemble G. tumida due to its tumid and elongate test (Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; Lam and Leckie, 2020a) but has a delicate test with thin keel (Bolli and Saunders, 1985). It showed a preference for the sinistral coiling. Lamb and Beard (1972) considered G. ungulata as a shallow-water, thin-walled growth form of G. tumida. Morard et al. (2015) confirmed the phylogenetic relationship of G. ungulata with G. tumida on the basis of molecular genetic data.
Shackleton and Vincent (1978) considered G. ungulata as a mixed-layer dweller, whereas Aze et al. (2011) assigned it open ocean thermocline habitat. It prefers tropical to subtropical latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011; Schiebel and Hemleben, 2017) but has a very sporadic appearance.
It showed sporadic and very low abundance in Hole U1474A in samples spanning Late Pliocene to recent.
Subgenus Hirsutella Bandy 1972
Type Species Rotalina hirsuta d'Orbigny 1839
Globorotalia (Hirsutella) bermudezi (Rögl and Bolli 1973)
(Plate P18, figures 1–5)
Plate P18. Globorotalia (H.) bermudezi, Globorotalia (H.) eastropacia, and Globorotalia (H.) hirsuta.
Synonym: Globorotalia bermudezi
Type species: Globorotalia bermudezi Rögl and Bolli, 1973
References: Rögl and Bolli (1973), Kennett and Srinivasan (1983), Loeblich and Tappan (1994), Aze et al. (2011)
Observed stratigraphic range: 361-U1474A-9H-4, 77–79 cm, to 1H-1, 0–2 cm
Remarks. G. bermudezi has been considered a junior synonym of G. scitula by many authors (Saito et al., 1981; Schiebel and Hemleben, 2017; Lam and Leckie, 2020a), although Kennett and Srinivasan (1983) and Aze et al. (2011) considered it as a distinct species. Brummer and Kučera (2022) consider G. bermudezi as the small extant forms of G. scitula with open umbilicus.
G. bermudezi is characterized by a very small, lobulate test, consisting of five to six chambers in the final whorl. The axial periphery is subangular, with umbilical side more convex than spiral side. The surface is smooth and irregularly perforated, a character that distinguishes G. bermudezi from G. scitula (Kennett and Srinivasan, 1983).
G. bermudezi is a thermocline dweller that ranges from tropical to temperate latitudes.
In the present work, it occurs in extremely low abundance and shows intermittent appearance in the samples restricted to the Quaternary Period.
Globorotalia (Hirsutella) eastropacia (Boltovskoy 1974)
(Plate P18, figures 6–11)
Basionym: Globorotalia hirsuta eastropacia
Synonym: Globorotalia eastropacia, Globorotalia theyeri (Fleisher 1974)
Type species: Globorotalia eastropacia Boltovskoy, 1974
References: Boltovskoy (1974), Fleisher (1974), Saito et al. (1981), Kennett and Srinivasan (1983), Vincent and Toumarkine (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-20H-1, 19–21 cm, to 1H-1, 0–2 cm
Remarks. G. eastropacia was earlier synonymized with G. theyeri. Kennett and Srinivasan (1983) considered G. eastropacia as a junior synonym of G. theyeri, but Brummer and Kučera (2022) showed that G. eastropacia has priority over G. theyeri and should be used as the correct name. G. eastropacia is characterized by large, thin test with a sharp outline and differs from G. margaritae in having a flat spiral side. There are four to five flaring chambers in the final whorl. The wall is smooth and finely perforate. There is a thin discontinuous keel on the periphery. The marginal keel and flaring chambers distinguish it from G. scitula.
G. eastropacia is a subthermocline dweller (Aze et al., 2011; Schiebel and Hemleben, 2017) and prefers low latitudes (Kennett and Srinivasan, 1983). It occurs rarely in Hole U1474A and only in extremely low abundance.
Globorotalia (Hirsutella) hirsuta (d'Orbigny 1839)
(Plate P18, figures 12–19)
Synonym: Globorotalia hirsuta, Globoquadrina patriciae McCulloch 1977
Type species: Globorotalia hirsuta d'Orbigny, 1839
References: d'Orbigny (1839), Banner and Blow (1960), Bolli and Bermúdez (1965), Kennett (1973), Hornibrook (1982), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Scott et al. (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-2H-3, 52–54 cm, to 1H-1, 0–2 cm
Remarks. G. hirsuta is characterized by a large test with an extremely convex and tumid spiral side and a concave umbilical side, giving it a spiroconical outline. It differs from G. margaritae in having a robust test with four crescent-shaped chambers in the final whorl and a pustulose surface. The wall is smooth and finely perforate (Aze et al., 2011).
Scott et al. (1990) mentioned that the spiroconical form tends to develop better in G. margaritae than G. hirsuta because of the extremely concave umbilical side in the latter.
G. hirsuta prefers thermocline habitat (Aze et al., 2011) and ranges from tropical to temperate latitudes (Kennett and Srinivasa, 1983; Schiebel and Hemleben, 2017). Kennett and Srinivasan (1983) assigned its first appearance age as Late Pliocene, whereas Wade et al. (2011) and Lam and Leckie (2020b) gave its a first appearance age in the Late Pleistocene. It is an extant species that is found often in plankton (Schiebel and Hemleben, 2017). In Hole U1474A, it is a commonly occurring species in the Late Quaternary samples, and is an important biostratigraphic marker.
Globorotalia (Hirsutella) margaritae (Bolli and Bermúdez 1965)
(Plate P19, figures 1–14)
Basionym: Globorotalia margaritae
Type species: Globorotalia margaritae Bolli and Bermúdez, 1965
References: Bolli and Bermúdez (1965), Kennett (1973), Hornibrook (1982), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Scott et al. (1990), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-21H-7, 5–7 cm, to 15H-4, 35–37 cm
Remarks. G. margaritae is an important Early Pliocene index fossil (Srinivasan and Kennett, 1981a). It is characterized by its spiroconical test (Scott et al., 1990), which has a convex spiral side and concave umbilical side. The test is delicate with five compressed chambers in the final whorl, and has a thin marginal keel. The surface is densely perforate and smooth, with pustules on the earlier chambers. Aperture is low slit, with a pronounced lip. The wall is nonspinose and smooth (Aze et al., 2011) and is sometimes covered with secondary calcite, which obscures the surface texture (Lam and Leckie, 2020a). Lam and Leckie (2020a) observed that the width of the last chamber is almost equal to the length of the shell, a feature that was also observed in several specimens in this work.
Three subspecies of G. margaritae have been identified: G. margaritae primitiva, G. margaritae margaritae, and G. margaritae evoluta (Cita, 1973; Bolli and Saunders, 1985). They have very subtle differences in their morphology, which makes it difficult to assign the morphotypes to these subspecies. In the present work, this scheme was not followed and all such forms were included in G. margaritae. It differs from G. scitula in having concavo-convex test with marginal keel.
G. margaritae is a thermocline dweller (Aze et al., 2011) that occurs from tropical to temperate latitudes (Kennett and Srinivasan, 1983). It occurs in quite low abundance in Hole U1474A.
Globorotalia (Hirsutella) scitula (Brady 1882)
(Plate P19, figures 15–22; Plate P20, figures 1–3)
Type species: Globorotalia scitula Brady, 1882
References: Brady (1882), Fleisher (1974), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Hornibrook et al. (1989), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. scitula is characterized by a medium-sized test with four to five crescentic chambers in the final whorl. The outline is lobulate and subcircular and completely lacks keel. The wall is porous and smooth, bearing pustules in the earlier chambers. The umbilical side is more porous than the spiral side (Schiebel and Hemleben, 2017). The slit-like aperture is umbilical-extraumbilical and bears a pronounced lip. G. scitula shows a preference for the dextral coiling.
It closely resembles Globorotalia (Hirsutella) bermudezi. Saito et al. (1981) have considered G. bermudezi as the junior synonym of G. scitula. Lam and Leckie (2020a) have included the concept of G. bermudezi in G. scitula, although they haven't synonymized the two species. On the other hand, Kennett and Srinivasan (1983) have considered G. bermudezi as a distinct species on the basis of its smaller size and more number of chambers.
G. scitula is a cosmopolitan species, more abundant in the mid latitudes (Schiebel and Hemleben, 2017) that prefers open ocean thermocline habitat (Aze et al., 2011). G. scitula occurs in extremely low abundance in Hole U1474A.
Subgenus Menardella Bandy 1972
Type Species Pulvinukina menardii Parker, Jones and Brady, 1865
Globorotalia (Menardella) limbata (Fornasini 1902)
(Plate P20, figures 4–7)
Type species: Rotalia limbata Fornasini, 1902
References: Fornasini (1902), Kennett and Srinivasan (1983), Norris (1998), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 9H-1, 79–81 cm
Remarks. G. limbata differs from G. menardii by having at least seven chambers in the final whorl, a less pronounced keel, and a lenticular outline in the axial view. The limbate spiral sutures are straight for most of their length, curving just before merging into the peripheral keel, giving a hockey stick shape (Blow, 1969). The sutures are thickened toward the periphery. The surface is smooth and densely perforate. G. limbata is a low-latitude dweller (Kennett and Srinivasan, 1983) and prefers open ocean thermocline (Aze et al., 2011). It is extremely low in abundance and shows irregular appearance in Hole U1474A.
Globorotalia (Menardella) menardii (Parker, Jones and Brady 1865)
(Plate P20, figures 7–18)
Type species: Globorotalia menardii Parker, Jones and Brady (1865)
References: Parker, Jones and Brady (1865), Kennett (1973), Hornibrook (1982), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Scott et al. (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. G. menardii is an extant species characterized by a low trochospiral, compressed test, with a circular outline and a prominent peripheral keel. It has five to six chambers in the final whorl, with strongly curved, limbate sutures on the spiral side merging into the keel. The wall is nonspinose and smooth and is densely perforate.
There is a lot of variation in the degree of convexity of the test, which has led to a lot of confusion in the identification. The test outline of G. menardii in the axial view ranges from flat, as seen in G. cultrata (e.g., Bolli and Saunders, 1985; Knappertbusch, 2007; Brummer and Kučera, 2022), to biconvex, as seen in G. menardii (Kennett and Srinivasan, 1983; Bolli and Saunders, 1985; Brummer and Kučera, 2022).
Another feature that adds to this confusion is the spiral sutures which are limbate. Kennett and Srinivasan (1983) mention that G. menardii has five to six wedge-shaped chambers with strongly curved raised spiral sutures. Brummer and Kučera (2022) have reported limbate sutures in G. menardii that are more thickened toward periphery, whereas those in G. cultrata show uniform thickness. Kennett and Srinivasan (1983) described Globorotalia limbata, which has six to eight chambers in the final whorl and limbate spiral sutures that are thickened where they merge into the keel. This is a cause for concern because if a six-chambered test of G. menardii and G. limbata are compared, there will be striking similarity between the two as per the description of Kennett and Srinivasan (1983) and Brummer and Kučera (2022). To avoid this confusion in the present work, G. menardii has been used for the tests with not more than six chambers and limbate spiral sutures which have a uniform thickness.
Past authors have reported a number of morphotypes of G. menardii. The Miocene to Early Pliocene forms were named G. menardii A, having five to six chambers in the final whorl, and G. menardii B, which had seven to seven and a half chambers in the final whorl (Bolli, 1970). Bolli and Saunders (1985) have illustrated two forms ranging from Pleistocene to Holocene: G. menardii menardii showing a robust, thick-walled test with more circular equatorial outline and G. menardii cultrata with a more delicate, thin test with more elongate equatorial outline. Another morphotype with peripheral spines was identified by Brady (1884) as G. menardii fimbriata. Both G. menardii cultrata and G. menardii fimbriata were often used as distinct species.
There has been a huge debate over the usage of the name G. menardii and G. cultrata that has been discussed at length by Banner and Blow (1960), Parker (1962), Stainforth et al. (1975), Knappertbusch (2007, 2016, 2022), and Brummer and Kučera (2022). Stainforth et al. (1975) recommended that the name G. menardii be retained for Miocene and younger menardiform species. This view was supported by Kennett and Srinivasan (1983). Knappertbusch (2007) identified four morphotypes of G. menardii, namely alpha, beta, gamma, and delta, of which the alpha morphotype was identified as G. menardii menardii, and the beta morphotype was considered G. menardii cultrata. A recent review by Brummer and Kučera (2022) argues for using G. menardii strictly for the forms that have gone extinct during Late Miocene and advocates usage of G. cultrata for the extant forms. These morphotypes have also been encountered in the present work but are very low in abundance. We agree with Kennett and Srinivasan (1983) for the use of the name G. menardii for all such forms.
G. menardii is a cosmopolitan species most frequent in tropical to subtropical waters (Kennett and Srinivasan, 1983; Schiebel and Hemleben, 2017). It prefers open ocean thermocline (Aze et al., 2011). G. menardii is a major component of surface and Pleistocene sediments due to its large size and occasionally thick calcite crust (Schiebel and Hemleben, 2017).
In the present study, G. menardii occurs in fairly high abundance during the Pliocene occasionally reaching >10% of the total assemblage.
Globorotalia (Menardella) miocenica (Palmer 1945)
(Plate P21, figures 1–3)
Plate P21. Globorotalia (M.) miocenica, Globorotalia (M.) multicamerata, Globorotalia (T.) crassaformis.
Basionym: Globorotalia menardii var. miocenica
Synonym: Globorotalia miocenica
Type species: Globorotalia miocenica Palmer, 1945
References: Palmer (1945), Kennett and Srinivasan (1983), Norris (1998)
Observed stratigraphic range: 361-U1474A-17H-4, 65–67 cm, to 11H-1, 41–43 cm (?)
Remarks. G. miocenica is characterized by a menardiform test with a flat spiral side and strongly convex umbilical side, giving it a planoconvex appearance in the axial view. It has six to seven chambers in the final whorl, with limbate spiral sutures.
It is a thermocline dweller (Aze et al., 2011) and preferred low latitudes (Kennett and Srinivasan, 1983; Aze et al., 2011).
G. miocenica is extremely rare in the present work.
Globorotalia (Menardella) multicamerata (Cushman and Jarvis 1930)
(Plate P21, figures 4–8)
Basionym: Globorotalia menardii var. multicamerata
Synonym: Globorotalia multicamerata
Type species: Globorotalia multicamerata Cushman and Jarvis, 1930
References: Cushman and Jarvis (1930), Postuma (1971), Kennett and Srinivasan (1983), Norris (1998), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 9H-6, 129–131 cm
Remarks. G. multicamerata has a large test with a circular outline, with eight to ten chambers, limbate spiral sutures, and smooth surface. It very closely resembles G. limbata but for number of chambers in the final whorl (eight to ten versus seven to eight). Sometimes the intergradation between these two species is so subtle that it becomes difficult to differentiate between them. An important characteristic is the umbilicus in form of a distinct circular pit (Kennett and Srinivasan, 1983).
This open ocean thermocline dweller (Aze et al., 2011) is found in tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983).
G. multicamerata is not a frequently occurring species and has been found in extremely low abundance in Hole U1474A.
Subgenus Truncorotalia Cushman and Bermúdez 1949
Type Species Rotalia truncatulinoides d'Orbigny, 1839
Globorotalia (Truncorotalia) crassaformis (Galloway and Wissler 1927)
(Plate P21, figures 9–21)
Basionym: Globigerina crassaformis
Synonym: Globorotalia crassaformis
Type species: Globorotalia crassaformis Galloway and Wissler, 1927
References: Galloway and Wissler (1927), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Scott et al. (1990), Bylinskaya (2005), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-20H-1, 19–21 cm, to 1H-1, 0–2 cm
Remarks. G. crassaformis very closely resembles G. puncticulata, but for its low, slit-like aperture, which in the latter case is high arched. It is characterized by a robust test with a subquadrate to subrounded equatorial profile and a lack of keel. The test has a flat to slightly convex spiral side, and the umbilical side is strongly convex, giving a planoconvex axial outline. It has four to four and a half chambers in the final whorl, sometimes five chambers in the variant G. crassaformis hessi. The wall is smooth, finely perforate, and heavily pustulose on both sides.
G. crassaformis is morphologically quite diverse and several names have been given for the different morphotypes (Saito et al., 1981). It was regarded as end form of evolutionary development by some authors, whereas certain others considered it as initial species from which other morphologically distinguishable taxa had developed (Bolli and Saunders, 1985). Several morphotypes of G. crassaformis have been identified: Globorotalia crassaformis oceanica Cushman and Bermúdez (1949), Globorotalia crotonensis Conato and Follador (1967), Globorotalia crassacrotonensis Conato and Follador (1967), Globorotalia crassaformis ronda Blow (1969), Globorotalia crassaformis viola Blow (1969), Globorotalia crassaformis hessi Bolli and Premoli Silva (1973), Globorotalia crassaconica Hornibrook (1981), Globorotalia crassaformis imbricata Krasheninnikov et al. (2002). Most of the authors have considered these as variants of G. crassaformis (Kennett and Srinivasan, 1983; Lam and Leckie, 2020a; Brummer and Kučera, 2022). Parker (1962) has considered G. crassaformis as the senior synonym of G. crassula. The common morphological feature in all the morphotypes/variants is the quadrate arrangement of the quasi-scituline shaped chambers (Cifelli and Glacon, 1979), suggesting a close phylogenetic relationship among them (Kennett and Srinivasan, 1983).
G. crassaformis extends from warm subtropical to temperate latitudes (Kennett and Srinivasan, 1983) and prefers open ocean subthermocline habitat (Aze et al., 2011; Brummer and Kučera, 2022). In Hole U1474A, G. crassaformis has occurred consistently from Pliocene to recent, although the abundance was not very high.
Globorotalia (Truncorotalia) tosaensis (Takayanagi and Saito 1962)
(Plate P22, figures 1–9)
Synonym: Globorotalia tosaensis
Type species: Globorotalia tosaensis Takayanagi and Saito, 1962
References: Takayanagi and Saito (1962), Kennett (1973), Keller (1978), Hornibrook (1982), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Scott et al. (1990), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-13H-7, 29–31 cm, to 3H-7, 30–32 cm
Remarks. G. tosaensis is an important biostratigraphic marker for the Pleistocene. It can be distinguished by the test with a circular outline, five chambers in the final whorl, and lack of keel. The umbilical side is extremely convex, giving it a planoconvex outline in the axial view. The wall is smooth and surface is perforate, sometimes with a crust that obscures the surface. It can be differentiated from G. crassaformis by circular outline and five chambers in the final whorl.
Blow (1969) considered the forms with and without crust as members of separate lineages, following which Rögl (1976) recognized four taxa branching from the crust-free G. tosaensis. These forms, however, were considered variants among the intergrading morphotypes (Berggren et al., 1967; Phillips et al., 1968; Hornibrook, 1976).
G. tosaensis is an open ocean subthermocline dweller (Aze et al., 2011), ranging from tropical to subtropical latitudes. In Hole U1474A, this species has a low abundance in the samples spanning Late Pliocene.
Globorotalia (Truncorotalia) truncatulinoides (d'Orbigny 1839)
(Plate P22, figures 10–20)
Basionym: Rotalia truncatulinoides
Synonym: Globorotalia truncatulinoides
Type species: Globorotalia truncatulinoides d'Orbigny, 1839
References: d'Orbigny (1839), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Cifelli and Scott (1986), Scott et al. (1990), Schiebel and Hemleben (2017), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-10H-1, 67–69 cm, to 1H-1, 0–2 cm
Remarks. G. truncatulinoides evolved from G. tosaensis by developing a marginal keel and more wide open umbilicus (Kennett and Srinivasan, 1983). The morphological features are very similar to G. tosaensis except for the presence of a marginal keel. The surface is encrusted with secondary calcite, obscuring the ultrastructure.
G. truncatulinoides is distinguished from G. tosaensis by the presence of a pronounced peripheral keel. In the present study, a few forms were encountered that had circular equatorial profile with five chambers, but the keel was present only on the final chamber. Such forms have been identified as G. tosaensis-truncatulinoides plexus forms (Kennett and Geitzenauer, 1969), but for the paleoceanographic reconstructions, such forms were counted as G. truncatulinoides. Some other forms like G. truncatulinoides pachytheca Blow (1969) and G. excelsa Sprovieri and Ruggieri (1980) were considered variants of G. truncatulinoides by Kennett and Srinivasan (1983) and Brummer and Kučera (2022), whereas Aze et al. (2011) considered them as separate species. Another species, G. cavernula Bé (1967), was considered a cryptic species of G. truncatulinoides by (Quillévéré et al., 2013), whereas Kennett and Srinivasan (1983) retained it as a distinct species.
G. truncatulinoides is considered to be an open ocean subthermocline dweller by Aze et al. (2011), whereas Schiebel and Hemleben (2005) suggest that of all extant species it lives in the deepest habitat. It extends from tropical to subtropical latitudes (Kennett and Srinivasan, 1983).
In Hole U1474A, G. truncatulinoides occurred in significant abundance and is an important proxy to track the migration of the Subtropical Front north and south during Quaternary in the Agulhas Current and Agulhas leakage region (Peeters et al., 2004).
Genus Neogloboquadrina Bandy, Frerichs and Vincent, 1967
Type Species Globigerina dutertrei d'Orbigny, 1839
Neogloboquadrina acostaensis (Blow 1971)
(Plate P23, figures 1–11)
Basionym: Globorotalia acostaensis
Synonym: Globorotalia (Turborotalia) acostaensis tegillata Brönnimann and Resig, 1971
Type species: Neogloboquadrina acostaensis Blow, 1959
References: Blow (1959), Postuma (1971), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 7H-2, 57–59 cm
Remarks. N. acostaensis has a small test, with five to five and a half chambers in the final whorl, and straight spiral and umbilical sutures. The wall is nonspinose and shows cancellate structure Aze et al., 2011; Lam and Leckie, 2020a), although Kennett and Srinivasan (1983) called it a reticulate. The last chamber has a characteristic lip extending up to the umbilicus.
Another subspecies Globorotalia acostaensis trochoidea was erected by Bizon and Bizon (1965) for forms with a slightly more convex spiral side but was later included in N. acostaensis for stratigraphic purposes (Bolli and Saunders, 1985).
N. acostaensis is an open ocean thermocline dweller (Aze et al., 2011), extending from tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983).
In Hole U1474A, it was found as commonly occurring species during the Pliocene, where it showed fairly high abundance, becoming rare during the Early Quaternary.
Neogloboquadrina humerosa (Takayanagi and Saito 1962)
(Plate P23, figures 12–14)
Basionym: Globorotalia humerosa
Synonym: Neogloboquadrina humerosa
Type species: Neogloboquadrina humerosa Takayanagi and Saito, 1962
References: Takayanagi and Saito (1962), Keller (1978), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 6H-3, 131–133 cm
Remarks. N. humerosa has morphological features, largely those of N. acostaensis, except for the larger test with six chambers and reduced/no apertural lip (Bolli and Saunders, 1985). It has a low arch, interiomarginal aperture that lacks umbilical tooth, which separates it from N. dutertrei.
N. humerosa is an open ocean thermocline dweller (Aze et al., 2011) that thrives in tropical–subtropical latitudes (Kennett and Srinivasan, 1983).
In Hole U1474A, it occurs irregularly and has low abundance.
Neogloboquadrina incompta (Cifelli 1961)
(Plate P23, figures 15–18; Plate P24, figures 1–3)
Basionym: Globigerina incompta
Synonym: Neogloboquadrina pachyderma (dextral)
Type species: Neogloboquadrina incompta Cifelli, 1961
References: Cifelli (1961), Kennett (1973), Kennett and Vella (1975), Keller (1978), Kennett and Srinivasan (1983), Darling et al. (2006), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 1H-1, 0–2 cm
Remarks. N. incompta is characterized by a test with quadrate outline, four to five chambers in the final whorl, and dextral coiling. It has a very similar morphology to N. pachyderma, but the two species are distinct on the basis of genetic data (Darling et al., 2006). N. pachyderma and N. incompta are of morphological similarity, except coiling; N. incompta is typically dextral, and N. pachyderma is characterized by sinistral coiling.
N. incompta was synonymized with N. pachyderma by Parker (1962), whereas another species was identified as N. pachyderma dextralis by Setty (1977), later lumped with N. incompta. Kipp (1976) used the name N. pachyderma-dutertrei intergrade (P/D intergrade), which was also included in the N. incompta (Brummer and Kučera, 2022).
Darling et al. (2006) showed that the coiling direction is a genetic trait, and not a morphological feature reflecting ecophenotypic variation. The two opposite coiling morphotypes appear to have diverged during the late Miocene, and they have distinctly different ecologies (Darling et al., 2006). The molecular genetic data, fossil evidence, biogeography, and ecology together call for separating the two coiling types of N. pachyderma as distinct species (Darling et al., 2000, 2006; André et al., 2014). Since then, N. incompta has been in popular use for naming the dextrally coiled form of the erstwhile N. pachyderma (dextral).
N. incompta is a thermocline dweller that thrives in cold water. In Hole U1474A it is an important indicator of the northward migration of the Subtropical Front. It shows very low abundance except the episodes of cooling when its often exceeds 5%–10% of the total faunal population.
Neogloboquadrina dutertrei (d'Orbigny 1839)
(Plate P24, figures 4–15)
Basionym: Globigerina dutertrei
Synonyms: Neogloboquadrina dutertrei, Neogloboquadrina eggeri Rhumbler (1901), Neogloboquadrina subcretacea Lomnicki (1901), Neogloboquadrina blowi Rögl and Bolli (1973), Neogloboquadrina himiensis Maiya et al. (1976), Neogloboquadrina kagaensis Maiya et al. (1976), Globigerina eggeriformis McCulloch (1977)
Type species: Neogloboquadrina dutertrei d'Orbigny, 1839
References: d'Orbigny (1839), Parker (1962), Kennett (1973), Keller (1980), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Schiebel and Hemleben (2017), Lam and Leckie (2020a), Brummer and Kučera (2022)
Observed stratigraphic range: 361-U1474A-21H-2, 123–125 cm, to 1H-1, 0–2 cm
Remarks. N. dutertrei is characterized by globose test with five to six chambers, slightly convex spiral side, and deep umbilicus with umbilical tooth. It is an important extant species that is used in paleoceanographic and paleoclimatic studies. It differs from N. acostaensis by its larger test and curved, radial sutures, and N. humerosa by its aperture and presence of umbilical teeth. The wall is nonspinose and cancellate (Aze et al., 2011).
Several attempts have been made to correlate variation in the morphological features of N. dutertrei and water-mass conditions. The high-spired forms were regarded as warm-water variants, and low-spired forms were considered to prefer cold water (Bradshaw, 1959; Bolli, 1970). Bandy et al. (1967) differentiated N. dutertrei in two subspecies on the basis of the umbilical tooth-like plates: (1) forms with umbilical plates, called as N. dutertrei dutertrei, and (2) forms without umbilical plates, termed as N. dutertrei subcretacea. Srinivasan and Kennett (1976) identified two kinds of surface ultramicrostructures restricted to different latitudinal ranges and identified two groups: N. dutertrei Group A, with relatively thin-walled test, high pore concentration, and pitted wall surface with microcrystals, and N. dutertrei Group B, with the characteristic rosette pattern formed by concentric arrangement of euhedral crystals on each chamber, characteristic of cool subtropical areas. Kipp (1976) classified forms with four to four and a half chambers in the final whorl and surface ultrastructure resembling that of N. dutertrei as N. pachy-duter (P-D intergrade). P-D intergrade was defined by Kipp (1976) as an informal group of pachyderma-dutertrei intergrades, which includes: (a) N. incompta that have more than four chambers per whorl when viewed from umbilical side (Parker, 1962), and (b) immature specimens of N. dutertrei without umbilical tooth (Boltovskoy, 1968). The P-D intergrades were later included in N. incompta (Kučera et al., 2005). These are characteristic of cooler waters and subtropical–subpolar latitudes and prefer a temperature ~20°C (Hilbrecht, 1996).
N. dutertrei is an important species in the tropical–subtropical zone, and sometimes also appears in the temperate zones during summer (Schiebel and Hemleben, 2017). It is considered as a salinity and productivity indicator in the tropical latitudes (Thunell, 1978; Cullen, 1981). Bijma et al. (1990) observed that under laboratory conditions, N. dutertrei can tolerate a wide range of salinity, 25–46 psu, and a temperature range of 13°–33°C. N. dutertrei is an important upwelling indicator (Thiede, 1983) and is indicative of mid to late phases of upwelling (Thunell and Sautter, 1992). Portilho-Ramos et al. (2017) used the relative abundance of N. dutertrei, N. pachyderma, and G. glutinata to track the intertropical convergence zone over Atlantic Ocean and observed the opposing pattern of abundance of N. dutertrei and G. glutinata during an upwelling episode.
In Hole U1474A, this species has fairly moderate abundance, with higher abundance during the Quaternary than during the Pliocene.
Genus Pulleniatina Cushman, 1927
Type Species Pullenia obliquiloculata Parker and Jones, 1865
Pulleniatina obliquiloculata (Parker and Jones 1865)
(Plate P25, figures 1–6)
Basionym: Pullenia sphaeroides var. obliquiloculata
Synonym: Pulleniatina obliquiloculata, Pulleniatina antillensis Bermúdez (1960), Pulleniatina trochospira Hartono (1964), Pulleniatina finalis Banner and Blow (1967), Pulleniatina okinawaensis Natori (1976)
Type species: Pulleniatina obliquiloculata Parker and Jones, 1865
Observed stratigraphic range: 361-U1474A-15H-1, 133–135 cm, to 1H-1, 0–2 cm
Remarks. P. obliquiloculata is an extant species characterized by a globose, streptospiral test, with a low arch aperture extending from the umbilical area to periphery and onto the spiral side (Kennett and Srinivasan, 1983). The wall is smooth due to a heavy cortex made of interlocking calcite plates. The apertural area is granular.
P. obliquiloculata has undergone several morphological variations. The test of P. finalis appears to show planispiral coiling due to an involute spiral side, with a very broadly rounded periphery, and high arch, entirely extraumbilical aperture (Bolli and Saunders, 1985). The variation in the coiling direction of P. obliquiloculata was an important event in the geological history (Saito, 1977; Pearson and Penny, 2021).
P. obliquiloculata is a tropical to warm subtropical species (Kennett and Srinivasan, 1983; Schiebel and Hemleben, 2017), and prefers thermocline habitat (Aze et al., 2011). It is an important species in the Pacific Ocean realm and is known as indicator of the Kuroshio Current (Lam and Leckie, 2020a).
In Hole U1474A, it is a regularly occurring species, with low to moderate abundance.
Pulleniatina primalis (Banner and Blow 1967)
(Plate P25, figures 7–16)
Basionym: Pulleniatina primalis
Synonyms: Pulleniatina praepulleniatina Brönnimann and Resig (1971)
Type species: Pulleniatina primalis Banner and Blow, 1967
References: Banner and Blow (1967), Parker (1962), Kennett (1973), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Chaisson and Leckie (1993), Lam and Leckie (2020a)
Observed stratigraphic range: 361-U1474A-25H-7, 26–28 cm, to 13H-2, 89–91 cm
Remarks. P. primalis is characterized by a medium test with streptospiral coiling, with four to five chambers in the final whorl. The surface is smooth and porcelaneous with a cortex. The distinguishing feature of P. primalis is its final chamber, which is more embracing, and the aperture, which is restricted to the umbilical side and doesn't reach the periphery of the previous whorl, unlike P. obliquiloculata, in which the aperture is extended over the umbilical area (Kennett and Srinivasan, 1983).
P. primalis was an open ocean thermocline dwelling species (Aze et al., 2011) and extended in the tropical to warm subtropical latitudes (Kennett and Srinivasan, 1983). It is an important biostratigraphic marker for the Pliocene in the lower latitudes.
In Hole U1474A, it was found to occur regularly in the Pliocene samples, in lower abundance.
Family HASTIGERINIDAE Bolli, Loeblich and Tappan, 1957
Genus Hastigerina Thomson, 1876
Type Species Nonionina pelagica d'Orbigny, 1839
Hastigerina pelagica (d'Orbigny 1839)
(Plate P26, figures 1–8)
Synonym: Hastigerina murrayi Thomson (1876)
Type species: Hastigerina pelagica d'Orbigny, 1839
References: d'Orbigny (1839), Saito et al. (1976), Kennett and Srinivasan (1983), Bolli and Saunders (1985), Loeblich and Tappan (1994), Schiebel and Hemleben (2017)
Observed stratigraphic range: 361-U1474A-25H-7, 26–29 cm, to 1H-1, 0–2 cm
Remarks. H. pelagica is distinguished by its almost planispiral test, with four to five rapidly enlarging chambers in the final whorl and characteristic triradiate spines. In spinose forms, it is the only species that produces entirely triradiate spines (Schiebel and Hemleben, 2017).
H. pelagica is found in tropical to temperate latitudes (Kennett and Srinivasan, 1983).
This species occurs regularly in Hole U1474A in extremely low abundance.
4. Acknowledgments
At the outset, we thank IODP and Kochi Core Center for providing the samples from Hole U1474A. V.P. Singh thanks the National Centre for Polar and Ocean Research (NCPOR) (India) for providing financial assistance through Project Grant NCPOR/IODP/E.3947/2021. R. Dwivedi thanks NCPOR for support in the form of a Junior Research Fellowship. S. Pathak thanks Indira Gandhi National Tribal University (IGNTU) (India) for financial support in the form of a Junior Research Fellowship. We extend our thanks to Dr. Rohit Pandey with the Department of Geology at Banaras Hindu University for extending the SEM facilities. V.P. Singh greatly appreciates the discussion on taxonomy with Professor Bridget Wade, Dr. Tracy Aze, Dr. Hellen Coxall, and Dr. Alessio Fabbrini during various meetings of the Neogene Quaternary Planktic Foraminiferal Working Group. The authors thankfully acknowledge the logistical support given by IGNTU.
References
Akers, W.H., 1955. Some planktonic foraminifera of the American Gulf Coast and suggested correlations with the Caribbean Tertiary. Journal of Paleontology, 29(4):647–664. http://www.jstor.org/stable/1300351
André, A., Quillévéré, F., Morard, R., Ujiié, Y., Escarguel, G., de Vargas, C., de Garidel-Thoron, T., and Douady, C.J., 2014. SSU rDNA divergence in planktonic foraminifera: molecular taxonomy and biogeographic implications. PloS One, 9(8):e104641. https://doi.org/10.1371/journal.pone.0104641
André, A., Weiner, A., Quillévéré, F., Aurahs, R., Morard, R., Douady, C.J., de Garidel-Thoron, T., Escarguel, G., de Vargas, C., and Kucera, M., 2013. The cryptic and the apparent reversed: lack of genetic differentiation within the morphologically diverse plexus of the planktonic foraminifer Globigerinoides sacculifer. Paleobiology, 39(1):21–39. https://doi.org/10.1666/0094-8373-39.1.21
Asano, K., 1957. The foraminifera from the adjacent seas of Japan, collected by the S. S. Soyo-maru, 1922-1930, Part 3: planktonic species. Science Reports of the Tohuku University, Series 2 (Geology), 28:1–26. https://tohoku.repo.nii.ac.jp/record/11946/files/KJ00004219275.pdf
Aurahs, R., Grimm, G.W., Hemleben, V., Hemleben, C., and Kucera, M., 2009. Geographical distribution of cryptic genetic types in the planktonic foraminifer Globigerinoides ruber. Molecular Ecology, 18(8):1692–1706. https://doi.org/10.1111/j.1365-294X.2009.04136.x
Aze, T., Ezard, T.H.G., Purvis, A., Coxall, H.K., Stewart, D.R.M., Wade, B.S., and Pearson, P.N., 2011. A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data. Biological Reviews, 86(4):900–927. https://doi.org/10.1111/j.1469-185X.2011.00178.x
Bandy, O.L., 1964. Cenozoic planktonic foraminiferal zonation. Micropaleontology, 10(1):1–17. https://doi.org/10.2307/1484621
Bandy, O.L., 1972. Origin and development of Globorotalia (Turborotalia) pachyderma (Ehrenberg). Micropaleontology, 18(3):294–318. https://doi.org/10.2307/1485010
Bandy, O.L., 1975. Messinian evaporite deposition and the Miocene/Pliocene boundary, Pasquasia-Capodarso Sections, Sicily. In Saito, T. and Burckle, L.H., Late Neogene Epoch Boundaries. New York (American Museum Natural History Micropaleontology Press), 49–63.
Banner, F.T., and Blow, W.H., 1960. Some primary types of species belonging to the superfamily Globigerinaceae. Contributions from the Cushman Foundation for Foraminiferal Research, 11(1):1–41.
Banner, F.T., and Blow, W.H., 1965. Two new taxa of the Globorotaliinae (Globigerinacea, Foraminfera) assisting determination of the late Miocene/middle Miocene boundary. Nature, 207(5004):1351–1354. https://doi.org/10.1038/2071351a0
Banner, F.T., and Blow, W.H., 1967. The origin, evolution and taxonomy of the foraminiferal genus Pulleniatina Cushman, 1927. Micropaleontology, 13(2):133–162. https://doi.org/10.2307/1484667
Barbieri, F., 1967. The foraminifera in the Pliocene section Vernasca-Castell Arquato including the "Piacenzian stratotype" (Piacenza Province). Memorie della Società italiana di scienze naturali e del Museo civico di storia naturale di Milano, 15(3):145–163.
Barbieri, F., 1971. Comments on some Pliocene stages and on the taxonomy of a few species of Globorotalia. L'Ateneo Parmense, Acta Nat., 7(1):3–24.
Barbieri, F., and Petrucci, F., 1967. La serie stratigraphique du Messinien au Calabrien dans la valle´e du T. Crostolo (Reggio Emilia-Italie Sept.). Memorie della Società italiana di scienze naturali e del Museo civico di storia naturale di Milano, 15(3):181–188.
Bé, A.W.H., 1967. Globorotalia cavernula, a new species of planktonic foraminifera from the Subantarctic Pacific Ocean. Contributions from the Cushman Foundation for Foraminiferal Research, 18:128–132.
Bé, A.W.H., 1977. An ecological, zoogeographic and taxonomic review of Recent planktonic foraminifera. In Ramsay, A.T.S., Oceanic Micropaleontology. (Acadmic Press), 1–100.
Bé, A.W.H., and Hamlin, W.H., 1967. Ecology of recent planktonic foraminifera: part 3: distribution in the North Atlantic during the summer of 1962. Micropaleontology, 13(1):87–106. https://doi.org/10.2307/1484808
Bé, A.W.H., and Hutson, W.H., 1977. Ecology of planktonic foraminifera and biogeographic patterns of life and fossil assemblages in the Indian Ocean. Micropaleontology, 23(4):369–414. https://doi.org/10.2307/1485406
Bé, A.W.H., and Tolderlund, D.S., 1971. Distribution and ecology of living planktonic foraminifera in surface waters of the Atlantic and Indian Oceans. In Funnel, B.M. and Riedel, W.R., The Micropaleontology of Oceans. Cambridge, United Kingdom (Cambridge University Press), 105–150.
Belford, D.J., 1962. Miocene and Pliocene planktonic foraminifera, Papua-New-Guinea. Bulletin, Bureau of Mineral Resources, Geology and Geophysics, Australia, 62(1):3–51.
Berggren, W.A., 1973. The Pliocene time scale: calibration of planktonic foraminiferal and calcareous nannoplankton zones. Nature, 243(5407):391–397. https://doi.org/10.1038/243391a0
Berggren, W.A., 1977. Late neogene planktonic foraminiferal biostratigraphy of the Rio Grande Rise (South Atlantic). Marine Micropaleontology, 2:265–313. https://doi.org/10.1016/0377-8398(77)90015-9
Berggren, W.A., Olsson, R.K., and Reyment, R.A., 1967. Origin and development of the foraminiferal genus Pseudohastigerina Banner and Blow, 1959. Micropaleontology, 13(3):265–288. https://doi.org/10.2307/1484830
Berggren, W.A., Kent, D.V., Flynn, J.J., and Van Couvering, J.A., 1985. Cenozoic geochronology. GSA Bulletin, 96(11):1407–1418. https://doi.org/10.1130/0016-7606(1985)96<1407:CG>2.0.CO;2
Berggren, W.A., Kent, D.V., Swisher, C.C., III, and Aubry, M.-P., 1995. A revised Cenozoic geochronology and chronostratigraphy. In Berggren, W.A., Kent, D.V., Aubry, M.-P., and Hardenbol, J., Geochronology, Time Scales and Global Stratigraphic Correlation. SEPM Special Publication, 54: 129–212. https://doi.org/10.2110/pec.95.04.0129
Bermúdez, P.J., 1960. Foraminíferos planctónicos del Golfo de Venezuela. Memoria de la Sociedad de Ciencias Naturales La Salle, 20:58–76.
Bermúdez, P.J., 1961. Contribución al estudio de las Globigerinidea de la región Caribe-Antillana (Paleoceno-Reciente). Memoria Tercer Congreso Geológico Venezolano, 3:1119–1393.
Bijma, J., Faber, W.W., and Hemleben, C., 1990. Temperature and salinity limits for growth and survival of some planktonic foraminifers in laboratory cultures. Journal of Foraminiferal Research, 20(2):95–116. https://doi.org/10.2113/gsjfr.20.2.95
Birch, H., Coxall, H.K., Pearson, P.N., Kroon, D., and O'Regan, M., 2013. Planktonic foraminifera stable isotopes and water column structure: Disentangling ecological signals. Marine Micropaleontology, 101:127–145. https://doi.org/10.1016/j.marmicro.2013.02.002
Bizon, J.-J., and Bizon, G., 1965. L'Helvétien et le Tortonien de la région de Parga (Epire continentale, Grèce). Revue de Micropaléontologie, 7:242–256.
Blow, W.H., 1956. Origin and evolution of the foraminiferal genus Orbulina d'Orbigny. Micropaleontology, 2(1):57–70. https://doi.org/10.2307/1484492
Blow, W.H., 1959. Age, correlation and biostratigraphy of the Upper Tocuyo (San Lorenzo) and Pozon formations, Eastern Falcon, Venezuela. Bulletins of American Paleontology, 39(178):59–251.
Blow, W.H., 1969. Late middle Eocene to Recent planktonic foraminiferal biostratigraphy. In Brönnimann, P. and Renz, H.H., Proceedings of the First International Conference on Planktonic Microfossils, Geneva, 1967. (Brill), 199–421. https://doi.org/10.1163/9789004616455_018
Blow, W.H., 1979. The Cainozoic Globigerinida: A study of the morphology, taxonomy, evolutionary relationships and stratigraphical distribution of some Globigerinida (mainly Globigerinacea): Leiden, The Netherlands (Brill).
Blow, W.H., and Banner, F.T., 1962. The Mid-Tertiary (Upper Eocene to Aquitanian) Globigerinacea. In Eames, F.E., Banner, F.T., Blow, W.H., and Clarke, W.J. (Eds.), Fundamentals of Mid-Tertiary stratigraphic correlation. Cambridge, UK (Cambridge University Press), 61–151.
Bolli, H.M., 1957. Planktonic Foraminifera from the Eocene Naver and San Fernando formations of Trinidad, B.W.I. United States National Museum Bulletin, 215:155–172. https://www.biodiversitylibrary.org/part/70800
Bolli, H.M., 1970. The foraminifera of Sites 23–31, Leg 4. In Bader, R.G., Gerard, R.D., et al., Initial Reports of the Deep Sea Drilling Project, 4: Washington, DC (U.S. Govt. Printing Office), 577–643. https://doi.org/10.2973/dsdp.proc.4.125.1970
Bolli, H.M., and Bermúdez, P.J., 1965. Zonation based on planktonic foraminifera of middle Miocene to Pliocene warm-water sediments. Boletin Informativo, Asociacion Venezolana de Geologia, Mineria y Petroleo, 8(5):121–149.
Bolli, H.M., and Premoli Silva, I., 1973. Oligocene to Recent planktonic foraminifera and stratigraphy of the Leg 15 sites in the Caribbean Sea. In Edgar, N.T., Saunders, J.B., et al., Initial Reports of the Deep Sea Drilling Project, 15: Washington, DC (U.S. Govt. Printing Office), 475–497. https://doi.org/10.2973/dsdp.proc.15.110.1973
Bolli, H.M., and Saunders, J.B., 1985. Oligocene to Holocene low latitude planktic foraminifera. In Bolli, H.M., Saunders, J.B., and Perch-Nielsen, K. (Eds.), Planktic Foraminifera, Calcareous Nannofossils and Calpionellids. Plankton Stratigraphy, 1: 155–262.
Boltovskoy, E., 1968. Living planktonic foraminifera of the eastern part of the tropical Atlantic. Revue de Micropaléontologie, 11(2):85–98.
Boltovskoy, E., 1974. Neogene planktonic foraminifera of the Indian Ocean (DSDP, Leg 26). In Davies, T.A., Luyendyk, B.P., et al., Initial Reports of the Deep Sea Drilling Project, 26: Washington, DC (U.S. Govt. Printing Office), 675–741. https://doi.org/10.2973/dsdp.proc.26.130.1974
Bradshaw, J.S., 1959. Ecology of living planktonic foraminifera in the North and Equatorial Pacific Ocean. Contributions from the Cushman Foundation for Foraminiferal Research, 10:25–64.
Brady, H.B., 1877. Supplementary note on the foraminifera of the Chalk (?) of the New Britain Group. Geological Magazine, 4(12):534–536. https://doi.org/10.1017/S0016756800150137
Brady, H.B., 1879. Notes on some of the Reticularian Rhizopoda of the "Challenger" Expedition. Quaternary Journal of Microscopical Science, s2-19(73):20–63. https://doi.org/10.1242/jcs.s2-19.73.20
Brady, H.B., 1882. Report on the Foraminifera. In Tizard, L., and Murray, J. (Eds.), Exploration of the Faröe Channel During the Summer of 1880, in Her Majesty's Ship Knight Errant, with Subsidiary Reports. Proceedings of the Royal Society of Edinburgh, 11: 708–717.
Brady, H.B., 1884. Report on the Foraminifera dredged by H.M.S. Challenger during the Years 1873-1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–18, 9:1–814. http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-22/html/doc.html
Brady, S., 1971. Size-depth variation in Cyclammina Cancellata Brady, Peru-Chile Trench. Antarctic Research Series: Antarctic Ocean, 15:309–331.
Brönnimann, P., 1951. The genus Orbulina d'Orbigny in the Oligo-Miocene of Trinidad, B.W. I. Contribution from the Cushman Foundation for foraminiferal research, 2(4):132–138.
Brönnimann, P., and Resig, J.M., 1971. A Neogene Globigerinacean biochronologic timescale of the southwestern Pacific. In Winterer, E.L., et al., Initial Reports of the Deep Sea Drilling Project, 7: Washington (U.S. Government Printing Office), 1235–1469. https://doi.org/10.2973/dsdp.proc.7.128.1971
Brummer, G.-J.A., and Kučera, M., 2022. Taxonomic review of living planktonic foraminifera. Micropaleontology, 41(1):29–74. https://doi.org/10.5194/jm-41-29-2022
Bylinskaya, M.E., 2005. Range and stratigraphic significance of the Globorotalia crassaformis plexus. Journal of Iberian Geology, 31(1):51–63.
Caley, T., Peeters, F.J.C., Biastoch, A., Rossignol, L., van Sebille, E., Durgadoo, J., Malaizé, B., Giraudeau, J., Arthur, K., and Zahn, R., 2014. Quantitative estimate of the paleo-Agulhas leakage. Geophysical Research Letters, 41(4):1238–1246. https://doi.org/10.1002/2014GL059278
Caron, D.A., Faber, W.W., and Bé, A.W.H., 1987. Growth of the spinose planktonic foraminifer Orbulina universa in laboratory culture and the effect of temperature on life processes. Journal of the Marine Biological Association of the United Kingdom, 67(2):343–358. https://doi.org/10.1017/S0025315400026655
Carpenter, W.B., Parker, W.K., and Jones, T.R., 1862. Introduction to the Study of the Foraminifera: London (The Ray Society). https://darwin-online.org.uk/converted/pdf/1862_Carpenter_Foraminifera_A3729.pdf
Caudri, C.M.B., 1934. Tertiary Deposits of Soemba: Amsterdam (H.J. Paris).
Chaisson, W.P., and Leckie, R.M., 1993. High-resolution Neogene planktonic foraminifer biostratigraphy of Site 806, Ontong Java Plateau (western equatorial Pacific). In Berger, W.H., Kroenke, L.W., Mayer, L.A., et al., Proceedings of the Ocean Drilling Program, Scientific Results, 130: College Station, TX (Ocean Drilling Program), 137–178. https://doi.org/10.2973/odp.proc.sr.130.010.1993
Chaisson, W.P., and Ravelo, A.C., 1997. Changes in upper water-column structure at Site 925, late Miocene-Pleistocene; planktonic foraminifer assemblages and isotopic evidence. In Shackleton, N.J., Curry, W.B., Richter, C., and Bralower, T.J. (Eds.), Proceedings of the Ocean Drilling Program; Scientific results, 154: College Station, TX (Ocean Drilling Program), 255–268. https://doi.org/10.2973/odp.proc.sr.154.105.1997
Chapman, F., Parr, W.J., and Collins, A.C., 1934. Tertiary foraminifera of Victoria, Australia–The Balcombian Deposits of Port Phillip. Part III. Zoological Journal of the Linnean Society, 38(262):553–577. https://doi.org/10.1111/j.1096-3642.1934.tb00996.x
Chapman, M.R., 2010. Seasonal production patterns of planktonic foraminifera in the NE Atlantic Ocean: Implications for paleotemperature and hydrographic reconstructions. Paleoceanography, 25(1). https://doi.org/10.1029/2008PA001708
Chaproniere, G.C.H., 1973. On the Origin of Globorotalia miotumida conomiozea Kennett, 1966. Micropaleontology, 19(4):461–468. https://doi.org/10.2307/1484908
Chaproniere, G.C.H., 1988. Globigerina woodi from the late Oligocene and early Miocene of southeastern Australia. Journal of Foraminiferal Research, 18(2):124–129. https://doi.org/10.2113/gsjfr.18.2.124
Chaproniere, G.C.H., Styzen, M.J., Sager, W.W., Nishi, H., Quinterno, P.J., and Abrahamsen, N., 1994. Late Neogene biostratigraphic and magnetostratigraphic synthesis, Leg 135. In Hawkins, J., Parson, L., Allan, J., et al., Proceedings of the Ocean Drilling Program, Scientific Results, 135: College Station, TX (Ocean Drilling Program), 857–877. https://doi.org/10.2973/odp.proc.sr.135.116.1994
Cifelli, R., 1961. Globigerina incompta, a new species of pelagic foraminifera from the North Atlantic. Contributions from the Cushman Foundation for Foraminiferal Research, 12(3):83–86.
Cifelli, R., and Glacon, G., 1979. New late Miocene and Pliocene occurrences of Globorotalia species from the North Atlantic; and a paleogeographic review. Journal of Foraminiferal Research, 9(3):210–227. https://doi.org/10.2113/gsjfr.9.3.210
Cifelli, R., and Scott, G., 1986. Stratigraphic record of the Neogene globorotalid radiation (planktonic foraminiferida). Smithsonian Contributions to Paleobiology, 58. https://repository.si.edu/bitstream/handle/10088/1980/SCtP-0058-Lo_res.pdf
Cita, M.B., 1973. Pliocene biostratigraphy and chronostratigraphy. In Ryan, W.B.F., Hsü, K. J., et al., Initial Reports of the Deep Sea Drilling Project, 13: Washington (U.S. Government Printing Office), 1343–1379. https://doi.org/10.2973/dsdp.proc.13.147-1.1973
Cita, M.B., and Ciaranfi, N., 1972. Studi sui Pliocene e sugli strati di passaggio dal Miocene al Pliocene, 2; a new species of Sphaeroidinella from late Neogene deep-sea Mediterranean sediments (DSDP Leg 13). Rivista Italiana Di Paleontologia E Stratigrafia, 79:693–710.
Conato, V., and Follador, U., 1967. Globorotalia crotonensis e Globorotalia crassacrotonensis nuove species del Pliocene Italiano. Bollettino della Societa Geologica Italiana, 84(3):555–563.
Coxall, H.K., and Spezzaferri, S., 2018. Taxonomy, biostratigraphy, and phylogeny of Oligocene Catapsydrax, Globorotaloides, and Protentelloides. In Wade, B.S., Olsson, R.K., Huber, B.T., and Berggren, W.A. (Eds.), Atlas of Oligocene planktonic Foraminifera. Special Publication - Cushman Foundation for Foraminiferal Research, 46: 79–124. https://www.ucl.ac.uk/earth-sciences/sites/earth-sciences/files/Chapter_4.pdf
Cullen, J.L., 1981. Microfossil evidence for changing salinity patterns in the Bay of Bengal over the last 20 000 years. Palaeogeography, Palaeoclimatology, Palaeoecology, 35:315–356. https://doi.org/10.1016/0031-0182(81)90101-2
Cushman, J.A., 1917. New species and varieties of foraminifera from the Philippines and adjacent waters [Scientific results of the Philippine cruise of the fisheries steamer "Albatross" 1907-1910–No. 35]. Proceedings of the United States National Museum, 51:651–662. https://www.biodiversitylibrary.org/page/7765033#page/1075
Cushman, J.A., 1918. Miocene foraminifera of the coastal plain of the United States. In Some Pliocene and Miocene foraminifera of the coastal plain of the United States, U.S. Geological Survey, Bulletin. 676: Washinigton (Government Printing Office), 39–73. https://pubs.usgs.gov/bul/0676/report.pdf
Cushman, J.A., 1919. Fossil foraminifera from the West Indies. In Vaughan, T.W., Contributions to the Geology and Paleontology of the West Indies. (Publications Carnegie Institution), 21–71.
Cushman, J.A., 1927. An outline of a re-classification of the Foraminifera. Contributions from the Cushman laboratory for foraminiferal research, 3:1–105.
Cushman, J.A., and Bermúdez, P.J., 1949. Some Cuban species of Globorotalia. Contributions from the Cushman laborotory for foraminiferal research, 25:26–45.
Cushman, J.A., and Jarvis, P.W., 1930. Miocene foraminifera from Buff Bay, Jamaica. Journal of Paleontology, 4(4):353–368. http://www.jstor.org/stable/1298001
Cushman, J.A., and Jarvis, P.W., 1936. Three new foraminifera from the Miocene, Bowden Marl, of Jamaica. Contributions from the Cushman laboratory for foraminiferal research, 12(1):3–5.
Cushman, J.A., and Renz, H.H., 1941. New Oligocene-Miocene foraminifera from Venezuela. Contributions from the Cushman laboratory for foraminiferal research, 17(1):1–27.
Darling, K.F., Kucera, M., Kroon, D., and Wade, C.M., 2006. A resolution for the coiling direction paradox in Neogloboquadrina pachyderma. Paleoceanography, 21(2):PA2011. https://doi.org/10.1029/2005PA001189
Darling, K.F., and Wade, C.M., 2008. The genetic diversity of planktic foraminifera and the global distribution of ribosomal RNA genotypes. Marine Micropaleontology, 67(3):216–238. https://doi.org/10.1016/j.marmicro.2008.01.009
Darling, K.F., Wade, C.M., Stewart, I.A., Kroon, D., Dingle, R., and Brown, A.J.L., 2000. Molecular evidence for genetic mixing of Arctic and Antarctic subpolar populations of planktonic foraminifers. Nature, 405(6782):43–47. https://doi.org/10.1038/35011002
de Vargas, C., Norris, R., Zaninetti, L., Gibb, S.W., and Pawlowski, J., 1999. Molecular evidence of cryptic speciation in planktonic foraminifers and their relation to oceanic provinces. Proceedings of the National Academy of Sciences of the United States of America, 96(6):2864–2868. https://doi.org/10.1073/pnas.96.6.2864
Deshayes, G.P., 1832. Encyclopedie methodique: Histoire Naturelle des Vers., Tome Second: Paris (Agasse Imprimeur).
Dollfus, G.F., 1905. Review of: Millett, F.W., Report on the Recent foraminifera of the Malay Archipelago. Revue Critique de Paléozoologie, 9:222–223.
d'Orbigny, A., 1846. Foraminifères fossiles du bassin tertiaire de Vienne (Autriche): Paris (Gide et Companie).
d'Orbigny, A.D., 1839. Foraminifères. In de la Sagra, R., Histoire physique, politique et naturelle de l'ile de Cuba. Paris (Arthus Bertrand).
d'Orbigny, A.D., 1826. Tableau méthodique de la classe des Céphalopodes. Annales des Sciences Naturelles, 7:245–314.
Duplessy, J.C., Bé, A.W.H., and Blanc, P.L., 1981. Oxygen and carbon isotopic composition and biogeographic distribution of planktonic foraminifera in the Indian Ocean. Palaeogeography, Palaeoclimatology, Palaeoecology, 33(1):9–46. https://doi.org/10.1016/0031-0182(81)90031-6
Dwivedi, R., Singh, V.P., Pathak, S., Mallick, K.R., and Nayak, P.K., 2024. Late Pliocene variability of the Agulhas Current: planktic foraminiferal records from southwest Indian Ocean. Journal of Geointerface, 3(2):103–112. https://doi.org/10.5281/zenodo.14279620
Earland, A., 1934. Foraminifera: Falklands sector of the Antarctic (excluding South Georgia), Part 3. Discovery Reports, 10:1–208.
Egger, J.G., 1893. Foraminiferen aus Meeresgrundproben, gelothet von 1874 bis 1876 von S.M. Sch. Gazelle: Munich (Verlag der K. Akademie).
El-Naggar, Z.R., 1971. On the classification, evolution and stratigraphical distribution of the Globigerinacea. In Farinacci, A., Proceedings of the Second Planktonic Conference, Roma 1970. 1: Rome (Edizioni Tecnoscienza), 421–476.
Fabbrini, A., Greco, M., Iacoviello, F., Kucera, M., Ezard, T.H.G., and Wade, B.S., 2023. Bridging the extant and fossil record of planktonic foraminifera: implications for the Globigerina lineage. Palaeontology, 66(6):e12676. https://doi.org/10.1111/pala.12676
Finlay, H.J., 1947. New Zealand Foraminifera: Key Species in Stratigraphy–No. 5. New Zealand journal of science and technology, B28:259–292.
Fleisher, R.L., 1974. Cenozoic planktonic foraminifera and biostratigraphy, Arabian Sea, Deep Sea Drilling Project, Leg 23A. In Whitmarsh, R.B., Weser, O.E., Ross, D.A., et al., Initial Reports of the Deep Sea Drilling Project, 23: Washington, DC (US Government Printing Office), 1001–1072. https://doi.org/10.2973/dsdp.proc.23.139.1974
Fornasini, C., 1902. Sinossi metodica, dei Foraminiferi sin qui rinvenuti nella sabbia del Lido di Rimini. Memorie della Reale Accademia delle Scienze dell'Istituto di Bologna, 5(10):1–68.
Fox, L.R., and Wade, B.S., 2013. Systematic taxonomy of early-middle Miocene planktonic Foraminifera from the equatorial Pacific Ocean: Integrated Ocean Drilling Program, Site U1338. Journal of Foraminiferal Research, 43(4):374–405. https://doi.org/10.2113/gsjfr.43.4.374
Frerichs, W.E., 1971. Planktonic foraminifera in the sediments of the Andaman Sea. Journal of Foraminiferal Research, 1(1):1–14. https://doi.org/10.2113/gsjfr.1.1.1
Galloway, J.J., and Wissler, S.G., 1927. Pleistocene foraminifera from the Lomita Quarry, Palos Verdes Hills, California. Journal of Paleontology, 1(1):35–87. http://www.jstor.org/stable/1298073
Gibbard, P.L., Head, M.J., Walker, M.J.C., and the Subcommission on Quaternary Stratigraphy, 2010. Formal ratification of the Quaternary system/period and the Pleistocene series/epoch with a base at 2.58 Ma. Journal of Quaternary Science, 25(2):96–102. https://doi.org/10.1002/jqs.1338
Gradstein, F.M., Ogg, J.G., and Smith, A.G., 2004. A Geologic Time Scale 2004: Cambridge, United Kingdom (Cambridge University Press). https://doi.org/10.1017/CBO9780511536045
Graham, R.M., and De Boer, A.M., 2013. The dynamical subtropical front. Journal of Geophysical Research: Oceans, 118(10):5676–5685. https://doi.org/10.1002/jgrc.20408
Hall, I.R., Hemming, S.R., LeVay, L.J., Barker, S.R., Berke, M.A., Brentegani, L., Caley, T., Cartagena-Sierra, A., Charles, C.D., Coenen, J.J., Crespin, J.G., Franzese, A.M., Gruetzner, J., Xibin, H., Hines, S.K.V., Jimenez Espejo, F.J., Just, J., Koutsodendris, A., Kubota, K., Lathika, N., Norris, R.D., Pereira dos Santos, T., Robinson, R., Rolison, J.M., Simon, M.H., Tangunan, D., van der Lubbe, J.J.L., Yamane, M., and Hucai, Z., 2017. Site U1474. In Hall, I.R., Hemming, S.R., LeVay, L.J., and the Expedition 361 Scientists, South African Climates (Agulhas LGM Density Profile). Proceedings of the International Ocean Discovery Program, 361: College Station, TX (International Ocean Discovery Program). https://doi.org/10.14379/iodp.proc.361.103.2017
Hartono, H.M.S., 1964. Coiling direction of Pulleniatina obliquiloculata trochospira n. var. and Globorotalia menardii. Bulletin Geological Survey of Indonesia, 1:5–12.
Hedberg, H.D., 1937. Foraminifera of the Middle Tertiary Carapita Formation of Northeastern Venezuela. Journal of Paleontology, 11(8):661–697. http://www.jstor.org/stable/1298440
Hemleben, C., and Olsson, R.K., 2006. Wall textures of Eocene planktonic foraminifera. In Pearson, P.N., Olsson, R.K., Huber, B.T., Hemleben, C. and Berggren, W.A., Atlas of Eocene Planktonic Foraminifera. 41: (Cushman Foundation for Foraminiferal Research).
Hemleben, C., Spindler, M., and Anderson, O.R., 1989. Modern Planktonic Foraminifera: New York (Springer-Verlag). https://doi.org/10.1007/978-1-4612-3544-6
Hilbrecht, H., 1996. Extant planktic foraminifera and the physical environment in the Atlantic and Indian Oceans. Mitteilungen aus dem Geologischen Institut der Eidgen, Technischen Hochschule und der Universität Zürich, Neue Folge, 300.
Hilgen, F.J., Abels, H.A., Iaccarino, S., Krijgsman, W., Raffi, I., Sprovieri, R., Turco, E., and Zachariasse, W.J., 2009. The Global Stratotype Section and Point (GSSP) of the Serravallian Stage (Middle Miocene). Episodes, 32(3):152–166. https://doi.org/10.18814/epiiugs/2009/v32i3/002
Hofker, J., 1976. La famille Turborotalitidae. Revue de Micropaléontologie, 19:47–53.
Hofker, J., Sr., 1956. Foraminifera of Santa Cruz and Thatcher Island, Virginia Archipelago, West Indies. Copenhagen University, Zoologisk Museum Spolia (Skrifler), 15:234.
Hornibrook, N.d.B., 1976. Globorotalia truncatulinoides and the Pliocene-Pleistocene boundary in Northern Hawkes Bay, New Zealand. In Takayanagi, Y., and Saito, T. (Eds.), Progress in Micropaleontology. New York (Micropaleontology Press), 83–102.
Hornibrook, N.d.B., 1981. Globorotalia (planktic Foraminiferida) in the Late Pliocene and Early Pleistocene of New Zealand. New Zealand Journal of Geology and Geophysics, 24(2):263-292. https://doi.org/10.1080/00288306.1981.10422717
Hornibrook, N.d.B., 1982. Late Miocene to Pleistocene Globorotalia (Foraminiferida) from DSDP Leg 29, Site 284, Southwest Pacific. New Zealand Journal of Geology and Geophysics, 25(1):83-99. https://doi.org/10.1080/00288306.1982.10422507
Hornibrook, N.d.B., Brazier, R.C., and Strong, C.P., 1989. Manual of New Zealand Permian to Pleistocene foraminiferal biostratigraphy. New Zealand Geological Survey Paleontological Bulletin, 56.
Huber, B.T., Petrizzo, M.R., Young, J.R., Falzoni, F., Gilardoni, S.E., Bown, P.R., and Wade, B.S., 2016. Pforams@microtax: a new online taxonomic database for planktonic foraminifera. Micropaleontology, 62(6):429–438. https://www.jstor.org/stable/26645533
Iaccarino, S., 1967. Les foraminifères du stratotype du Tabianien (Pliocéne inférieur) de Tabiano Bagni (Parme). Memorie della Società italiana di scienze naturali Milano, 16(3):165–180.
Iaccarino, S., 1985. Mediterranean Miocene and Pliocene planktonic foraminifera. In Bolli, H.M., Saunders, J.B., and Perch-Nielsen, K. (Eds.), Plankton stratigraphy. 1: New York (Cambridge University Press).
Jayan, A.K., Sijinkumar, A.V., and Nath, B.N., 2021. Paleoceanographic significance of Globigerinoides ruber (white) morphotypes from the Andaman Sea. Marine Micropaleontology, 165:101996. https://doi.org/10.1016/j.marmicro.2021.101996
Jedlitschka, H., 1934. Über Candorbulina, eine neue Foraminiferen-Gattung und zwei neue Candeina Arten. Verhandlungen des Naturforschenden Vereins in Brünn, 65:17–26. https://www.zobodat.at/pdf/Verh-naturf-Ver-Bruenn_65_0017-0026.pdf
Jenkins, D.G., 1960. Planktonic foraminifera from the Lakes Entrance Oil Shaft, Victoria, Australia. Micropaleontology, 6(4):345–371. https://doi.org/10.2307/1484217
Jenkins, D.G., 1968. Variations in the numbers of species and subspecies of planktonic Foraminiferida as an indicator of New Zealand Cenozoic paleotemperatures. Palaeogeography, Palaeoclimatology, Palaeoecology, 5(3):309–313. https://doi.org/10.1016/0031-0182(68)90073-4
Jenkins, D.G., 1971. New Zealand Cenozoic planktonic foraminifera. New Zealand Geological Survey Paleontological Bulletin, 42.
Jenkins, D.G., 1985. Southern mid-latitude Paleocene to Holocene planktic foraminifera. In Bolli, H.M., Saunders, J.B., and Perch-Nielsen, K. (Eds.), Plankton stratigraphy. Cambridge (Cambridge University Press), 263–282.
Jenkins, D.G., and Srinivasan, M.S., 1986. Cenozoic planktonic foraminifers from the equator to the subantarctic of the Southwest Pacific. In Kennett, J.P., von der Borch, C. C., et al., Initial Reports of the Deep Sea Drilling Project, 90: Washington, DC (US Government Printing Office), 795–834. https://doi.org/10.2973/dsdp.proc.90.113.1986
Kaushik, T., Singh, A.K., and Sinha, D.K., 2020. Late Neogene–Quaternary planktic foraminiferal biostratigraphy and biochronology from ODP Site 807A, Ontong Java Plateau, Western Equatorial Pacific. Journal of Foraminiferal Research, 50(2):111–127. https://doi.org/10.2113/gsjfr.50.2.111
Keijzer, F.G., 1945. Outline of the geology of the eastern part of the province of Oriente, Cuba (E of 76o W.L.), with notes on the geology of other parts of the Island, Series 2, No. 6: Utrecht (De Vliegende Hollander).
Keller, G., 1978. Morphologic variation of Neogloboquadrina pachyderma (Ehrenberg) in sediments of the marginal and central Northeast Pacific Ocean and paleoclimatic interpretation. Journal of Foraminiferal Research, 8(3):208–224. https://doi.org/10.2113/gsjfr.8.3.208
Keller, G., 1980. Early to middle Miocene planktonic foraminiferal datum levels of the equatorial and subtropical Pacific. Micropaleontology, 26(4):372–391. https://doi.org/10.2307/1485351
Keller, G., 1981. Origin and evolution of the genus Globigerinoides in the early Miocene of the northwestern Pacific, DSDP Site 292. Micropaleontology, 27(3):293–304. https://doi.org/10.2307/1485239
Keller, G., 1985. Depth stratification of planktonic foraminifers in the Miocene ocean. In Kennett, J.P., The Miocene Ocean: Paleoceanography and Biogeography. Memoirs - Geological Society of America, 163: 177–195. https://doi.org/10.1130/MEM163-p177
Kennett, J.P., 1966. The Globorotalia crassaformis bioseries in north Westland and Marlborough, New Zealand. Micropaleontology, 12(2):235–245. https://doi.org/10.2307/1484711
Kennett, J.P., 1973. Middle and late Cenozoic planktonic foraminiferal biostratigraphy of the Southwest Pacific—DSDP Leg 21. In Burns, R.E., Andrews, J.E., et al., Initial Reports of the Deep Sea Drilling Project, 21: Washington, DC (US Government Printing Office), 575–639. https://doi.org/10.2973/dsdp.proc.21.117.1973
Kennett, J.P., and Srinivasan, M.S., 1983. Neogene Planktonic Foraminifera: A Phylogenetic Atlas: London (Hutchinson Ross).
Kennett, J.P., and Vella, P., 1975. Late Cenozoic planktonic foraminifera and paleoceanography at DSDP Site 284 in the cool subtropical South Pacific. In Kennett, J.P., Houtz, R.E., et al., Initial Reports of the Deep Sea Drilling Project, 29: Washington, DC (US Government Printing Office), 769–799. https://doi.org/10.2973/dsdp.proc.29.119.1975
King, D.J., Wade, B.S., Liska, R.D., and Miller, C.G., 2020. A review of the importance of the Caribbean region in Oligo-Miocene low latitude planktonic foraminiferal biostratigraphy and the implications for modern biogeochronological schemes. Earth-Science Reviews, 202:102968. https://doi.org/10.1016/j.earscirev.2019.102968
Kipp, N.G., 1976. New transfer function for estimating past sea-surface conditions from sea-bed distribution of planktonic foraminiferal assemblages in the North Atlantic. In Cune, R.M. and Hays, J.D., Investigation of Late Quaternary Paleoceanography and Paleoclimatology. 145: (Geological Society of America), 3–41. https://doi.org/10.1130/MEM145-p3
Knappertsbusch, M., 2007. Morphological variability of Globorotalia menardii (planktonic foraminifera) in two DSDP cores from the Caribbean Sea and the Eastern Equatorial Pacific. Carnets de Géologie, 7:CG2007_A2004. http://paleopolis.rediris.es/cg/07/A04/
Knappertsbusch, M., 2016. Evolutionary prospection in the Neogene planktic foraminifer Globorotalia menardii and related forms from ODP Hole 925B (Ceara Rise, western tropical Atlantic): evidence for gradual evolution superimposed by long distance dispersal? Swiss Journal of Palaeontology, 135(2):205–248. https://doi.org/10.1007/s13358-016-0113-6
Knappertsbusch, M., 2022. Morphological evolution of menardiform globorotalids at Western Pacific Warm Pool ODP Hole 806C (Ontong-Java Plateau). Revue de Micropaléontologie, 74:100608. https://doi.org/10.1016/j.revmic.2022.100608
Kučera, M., 1998. Biochronology of the mid-Pliocene Sphaeroidinella event. Marine Micropaleontology, 35(1):1–16. https://doi.org/10.1016/S0377-8398(98)00016-4
Kučera, M., Weinelt, M., Kiefer, T., Pflaumann, U., Hayes, A., Weinelt, M., Chen, M.-T., Mix, A.C., Barrows, T.T., Cortijo, E., Duprat, J., Juggins, S., and Waelbroeck, C., 2005. Reconstruction of sea-surface temperatures from assemblages of planktonic foraminifera: multi-technique approach based on geographically constrained calibration data sets and its application to glacial Atlantic and Pacific Oceans. Quaternary Science Reviews, 24(7):951–998. https://doi.org/10.1016/j.quascirev.2004.07.014
Lam, A.R., and Leckie, R.M., 2020a. Late Neogene and Quaternary diversity and taxonomy of subtropical to temperate planktic foraminifera across the Kuroshio Current Extension, northwest Pacific Ocean. Micropaleontology, 66(3):177–268. https://doi.org/10.47894/mpal.66.3.01
Lam, A.R., and Leckie, R.M., 2020b. Subtropical to temperate late Neogene to Quaternary planktic foraminiferal biostratigraphy across the Kuroshio Current Extension, Shatsky Rise, northwest Pacific Ocean. PloS One, 15(7):e0234351. https://doi.org/10.1371/journal.pone.0234351
Lamb, J.L., 1969. Planktonic foraminiferal datums and late Neogene epoch boundaries in the Mediterranean, Caribbean, and Gulf of Mexico. Gulf Coast Association of Geological Societies Transactions, 19:559–578. https://archives.datapages.com/data/gcags/data/019/019001/0559.html
Lamb, J.L., and Beard, J.H., 1972. Late Neogene planktonic foraminifers in the Caribbean, Gulf of Mexico, and Italian stratotypes: (The Paleontological Institute, The University of Kansas). https://hdl.handle.net/1808/3832
Latas, M., Pearson, P.N., Poole, C.R., Fabbrini, A., and Wade, B.S., 2023. Globigerinoides rublobatus – a new species of Pleistocene planktonic foraminifera. Journal of Micropalaeontology, 42(2):57–81. https://doi.org/10.5194/jm-42-57-2023
LeRoy, L.W., 1944. Miocene foraminifera from Sumatra and Java, Netherlands East Indies, Part 2. Small foraminifera from the Miocene of west Java, Netherlands East Indies. Quarterly of the Colorado School of Mines, 39(3):71–113.
Lessa, D., Morard, R., Jonkers, L., Venancio, I.M., Reuter, R., Baumeister, A., Albuquerque, A.L., and Kucera, M., 2020. Distribution of planktonic foraminifera in the subtropical South Atlantic: depth hierarchy of controlling factors. Biogeosciences, 17(16):4313–4342. https://doi.org/10.5194/bg-17-4313-2020
Li, Q., and McGowran, B., 2000. Miocene foraminifera from Lakes Entrance Oil Shaft, Gippsland, southeastern Australia. Memoirs of the Association of Australasian Palaeontologists, 22:1–142. http://hdl.handle.net/2440/13607
Lipps, J.H., 1964. Miocene planktonic Foraminifera from Newport Bay California. Tulane Studies in Geology, 2(4):109–133. https://journals.tulane.edu/tsgp/article/view/392
Loeblich, A.R., and Tappan, H.N., 1994. Foraminifera of the Sahul Shelf and Timor Sea. Cushman Foundation for Foraminiferal Research, Special Publication, 31.
Lomnicki, J.R., von, 1901. Einige bemerkungen zum aufsatze: die Miocänen foraminiferen in der umgebung von Kolomea. Verhandlungen des naturforschenden Vereines Brünn, 39:15–18.
Lutjeharms, J.R.E., 2006. The Agulhas Current: Berlin (Springer-Verlag). https://doi.org/10.1007/3-540-37212-1
Lutjeharms, J.R.E., 2009. Agulhas Current. In Steele, J.H., Thorpe, S. A., and Turekian, K. K., Ocean Currents. Encyclopedia of Ocean Sciences, 2nd Edition: London (Academic Press), 174–186.
Maiya, S., Saito, T., and Sato, T., 1976. Late Cenozoic planktonic foraminiferal biostratigraphy of Northwest Pacific sedimentary sequences. In Takayanagi, Y., and Saito, T. (Eds.), Progress in Micropaleontology. New York (Micropaleontology Press), 395–422.
Malmgren, B.A., and Kennett, J.P., 1981. Phyletic gradualism in a Late Cenozoic planktonic foraminiferal lineage; DSDP Site 284, southwest Pacific. Paleobiology, 7(2):230–240. https://doi.org/10.1017/S0094837300004000
Malmgren, B.A., Kucera, M., and Ekman, G., 1996. Evolutionary changes in supplementary apertural characteristics of the late Neogene Sphaeroidinella dehiscens lineage (planktonic Foraminifera). Palaios, 11(2):192–206. https://doi.org/10.2307/3515070
McCulloch, I.A., 1977. Qualitative Observations on Recent Foraminiferal Tests with Emphasis on the Eastern Pacific: Los Angeles (University of Southern California, Publications).
Meilland, J., Cornuault, P., Morard, R., Brummer, G.-J.A., and Kucera, M., 2022. Identification guide to extant planktonic foraminifera. Part 1: Family Candeinidae and genera Berggrenia, Bolivina, Dentigloborotalia, and Neogallitellia. ICES Identification Leaflets for Plankton, 196:1–22. https://doi.org/10.17895/ices.pub.7643
Morard, R., Füllberg, A., Brummer, G.-J.A., Greco, M., Jonkers, L., Wizemann, A., Weiner, A.K.M., Darling, K., Siccha, M., Ledevin, R., Kitazato, H., de Garidel-Thoron, T., de Vargas, C., and Kucera, M., 2019. Genetic and morphological divergence in the warm-water planktonic foraminifera genus Globigerinoides. PloS One, 14(12):e0225246. https://doi.org/10.1371/journal.pone.0225246
Morard, R., Quillévéré, F., Douady, C.J., de Vargas, C., de Garidel-Thoron, T., and Escarguel, G., 2011. Worldwide genotyping in the planktonic foraminifer Globoconella inflata: implications for life history and paleoceanography. PloS One, 6(10):e26665. https://doi.org/10.1371/journal.pone.0026665
Morard, R., Quillévéré, F., Escarguel, G., de Garidel-Thoron, T., de Vargas, C., and Kucera, M., 2013. Ecological modeling of the temperature dependence of cryptic species of planktonic Foraminifera in the Southern Hemisphere. Palaeogeography, Palaeoclimatology, Palaeoecology, 391:13–33. https://doi.org/10.1016/j.palaeo.2013.05.011
Morard, R., Quillévéré, F., Escarguel, G., Ujiie, Y., de Garidel-Thoron, T., Norris, R.D., and de Vargas, C., 2009. Morphological recognition of cryptic species in the planktonic foraminifer Orbulina universa. Marine Micropaleontology, 71(3):148–165. https://doi.org/10.1016/j.marmicro.2009.03.001
Morard, R., Reinelt, M., Chiessi, C.M., Groeneveld, J., and Kucera, M., 2016. Tracing shifts of oceanic fronts using the cryptic diversity of the planktonic foraminifera Globorotalia inflata. Paleoceanography, 31(9):1193–1205. https://doi.org/10.1002/2016PA002977
Naidu, P.D., Babu, C.P., and Rao, Ch.M., 1992. The upwelling record in the sediments of the western continental margin of India. Deep Sea Research Part A. Oceanographic Research Papers, 39(3):715–723. https://doi.org/10.1016/0198-0149(92)90097-D
Naidu, P.D., and Malmgren, B.A., 1996. A high-resolution record of Late Quaternary upwelling along the Oman Margin, Arabian Sea based on planktonic foraminifera. Paleoceanography, 11(1):129–140. https://doi.org/10.1029/95PA03198
Natland, M.L., 1938. New species of foraminifera from off the west coast of North America and from the later Tertiary of the Los Angeles basin. Bulletin of the Scripps Institution of Oceanography of the University of California, technical series, 4(5):137–163.
Norris, R.D., 1998. Planktonic foraminifer biostratigraphy: Eastern Equatorial Atlantic. In Mascle, J., Lohmann, G.P., and Moullade, M. (Eds.), Proceedings of the Ocean Drilling Program, Scientific Results, 159: College Station, TX (Ocean Drilling Program), 445–479. https://doi.org/10.2973/odp.proc.sr.159.036.1998
Numberger, L., Hemleben, C., Hoffmann, R., Mackensen, A., Schulz, H., Wunderlich, J.-M., and Kucera, M., 2009. Habitats, abundance patterns and isotopic signals of morphotypes of the planktonic foraminifer Globigerinoides ruber (d'Orbigny) in the eastern Mediterranean Sea since the Marine Isotopic Stage 12. Marine Micropaleontology, 73(1):90–104. https://doi.org/10.1016/j.marmicro.2009.07.004
Ogg, J.G., 2020. Geomagnetic Polarity Time Scale. In Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G. (Eds.), Geologic Time Scale 2020. Amsterdam (Elsevier), 159–192. https://doi.org/10.1016/B978-0-12-824360-2.00005-X
Orsi, A.H., Whitworth, T., and Nowlin, W.D., 1995. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Research, Part I: Oceanographic Research Papers, 42(5):641–673. https://doi.org/10.1016/0967-0637(95)00021-W
Ortiz, J.D., Mix, A.C., Rugh, W., Watkins, J.M., and Collier, R.W., 1996. Deep-dwelling planktonic foraminifera of the northeastern Pacific Ocean reveal environmental control of oxygen and carbon isotopic disequilibria. Geochimica et Cosmochimica Acta, 60(22):4509–4523. https://doi.org/10.1016/S0016-7037(96)00256-6
Ottens, J.J., 1992. April and August Northeast Atlantic surface water masses reflected in planktic foraminifera. Netherlands Journal of Sea Research, 28(4):261–283. https://doi.org/10.1016/0077-7579(92)90031-9
Palmer, D.K., 1945. Notes on the foraminifera from Bowden, Jamaica. Bulletins of American Paleontology, 29(115):1–82.
Parker, F.L., 1958. Eastern Mediterranean foraminifera. Reports of the Swedish Deep-Sea Expedition, Sediment Cores from the Mediterranean Sea and the Red Sea, 8(4):217–285.
Parker, F.L., 1962. Planktonic foraminiferal species in Pacific sediments. Micropaleontology, 8(2):219–254. https://doi.org/10.2307/1484745
Parker, F.L., 1967. Late Tertiary biostratigraphy (planktonic foraminifera) of tropical Indo-Pacific deep-sea cores. Bulletins of American Paleontology, 52:155–208.
Parker, W.K., and Jones, T.R., 1865. On some foraminifera from the North Atlantic and Arctic Oceans, including Davis Strait and Baffin Bay. Philosophical Transactions of the Royal Society of London, 155:325–441. http://www.jstor.org/stable/112038
Pearson, P.N., 1995. Planktonic foraminifer biostratigraphy and the development of pelagic caps on guyots in the Marshall Islands group. In Haggerty, J.A., Premoli Suva, L, Rack, R, and McNutt, M.K. (Eds.), Proceedings of the Ocean Drilling Program, Scientific Results, 144: College Station, TX (Ocean Drilling Program), 21–59. https://doi.org/10.2973/odp.proc.sr.144.013.1995
Pearson, P.N., and Penny, L., 2021. Coiling directions in the planktonic foraminifer Pulleniatina: a complex eco-evolutionary dynamic spanning millions of years. PloS One, 16(4):e0249113. https://doi.org/10.1371/journal.pone.0249113
Pearson, P.N., Shackleton, N.J., and Hall ,M.A., 1997. Stable isotopic evidence for the sympatric divergence of Globigerinoides trilobus and Orbulina universa (planktonic foraminifera). Journal of the Geological Society, 154(2):295–302. https://doi.org/10.1144/gsjgs.154.2.0295
Pearson, P.N., and Wade, B.S., 2009. Taxonomy and stable isotope paleoecology of well-preserved planktonic foraminifera from the uppermost Oligocene of Trinidad. Journal of Foraminiferal Research, 39(3):191–217. https://doi.org/10.2113/gsjfr.39.3.191
Pearson, P.N., Wade, B.S., and Huber, B.T., 2018. Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerinitidae (Dipsidripella, Globigerinita, and Tenuitella). In Wade, B.S., Olsson, R.K., Pearson, P.N., Huber, B.T. and Berggren, W.A., Atlas of Oligocene planktonic Foraminifera. Cushman Foundation Special Publication, 46: (Cushman Foundation for Foraminiferal Research), 429–458. https://pubs.geoscienceworld.org/cushmanfoundation/books/edited-volume/2271/chapter-abstract/126275385/TAXONOMY-BIOSTRATIGRAPHY-AND-PHYLOGENY-OF
Peeters, F.J.C., Acheson, R., Brummer, G.-J.A., de Ruijter, W.P.M., Schneider, R.R., Ganssen, G.M., Ufkes, E., and Kroon, D., 2004. Vigorous exchange between the Indian and Atlantic Oceans at the end of the past five glacial periods. Nature, 430(7000):661–665. https://doi.org/10.1038/nature02785
Perconig, E., 1968. Nuove specie di foraminiferi planctonici della Sezione di Carmona (Andalusia, Spagna). Committee Mediterranean Neogene Stratigraphy, 35(3):219–232.
Poole, C.R., and Wade, B.S., 2019. Systematic taxonomy of the Trilobatus sacculifer plexus and descendant Globigerinoidesella fistulosa (planktonic foraminifera). Journal of Systematic Palaeontology, 17(23):1989–2030. https://doi.org/10.1080/14772019.2019.1578831
Portilho-Ramos, R.C., Chiessi, C.M., Zhang, Y., Mulitza, S., Kucera, M., Siccha, M., Prange, M., and Paul, A., 2017. Coupling of equatorial Atlantic surface stratification to glacial shifts in the tropical rainbelt. Scientific Reports, 7(1):1561. https://doi.org/10.1038/s41598-017-01629-z
Postuma, J.A., 1971. Manual of Planktonic Foraminifera: Amsterdam (Elsevier).
Quillévéré, F., Morard, R., Escarguel, G., Douady, C.J., Ujiié, Y., de Garidel-Thoron, T., and de Vargas, C., 2013. Global scale same-specimen morpho-genetic analysis of Truncorotalia truncatulinoides: A perspective on the morphological species concept in planktonic foraminifera. Palaeogeography, Palaeoclimatology, Palaeoecology, 391:2–12. https://doi.org/10.1016/j.palaeo.2011.03.013
Raffi, I., Wade, B.S., Pälike, H., Beu, A.G., Cooper, R., Crundwell, M.P., Krijgsman, W., Moore, T., Raine, I., Sardella, R., and Vernyhorova, Y.V., 2020. The Neogene Period. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G. (Eds.), Geologic Time Scale 2020. (Elsevier), 1141–1215. https://doi.org/10.1016/B978-0-12-824360-2.00029-2
Reuss, A.E., 1850. Neue Foraminiferen aus den Schichten des österreichischen Tertiärbeckens. Denkschriften der Kaiserlichen Akademie der Wissenschaften, 1:365–390.
Rhumbler, L., 1901. Nordische Plankton-Foraminiferen. In Brandt, K., Nordische Plankton. 14, 1: Kiel (Lipsius und Tischer), 1–32.
Robbins, L.L., 1988. Environmental significance of morphologic variability in open-ocean versus ocean-margin assemblages of Orbulina universa. Journal of Foraminiferal Research, 18(4):326–333. https://doi.org/10.2113/gsjfr.18.4.326
Rögl, F., 1976. Late Cretaceous to Pleistocene foraminifera from the Southeast Pacific Basin, DSDP Leg 35. In Hollister, C.D., Craddock, C., et al., Initial Reports of the Deep Sea Drilling Project, 35: Washington (U.S. Government Printing Office), 539–555. https://doi.org/10.2973/dsdp.proc.35.133.1976
Rögl, F., and Bolli, H.M., 1973. Holocene to Pleistocene planktonic foraminifera of Leg 15, Site 147 (Cariaco Basin (trench), Caribbean Sea) and their climatic interpretation. In Edgar, N.T., Saunders, J. B., et al., Initial Reports of the Deep Sea Drilling Project, 15: Washington, DC (U.S. Government Printing Office), 553–615. https://doi.org/10.2973/dsdp.proc.15.113.1973
Rossignol, L., Eynaud, F., Bourget, J., Zaragosi, S., Fontanier, C., Ellouz-Zimmermann, N., and Lanfumey, V., 2011. High occurrence of Orbulina suturalis and "Praeorbulina-like specimens" in sediments of the northern Arabian Sea during the Last Glacial Maximum. Marine Micropaleontology, 79(3–4):100–113. https://doi.org/10.1016/j.marmicro.2011.01.006
Saito, T., 1977. Late Cenozoic planktonic foraminiferal datum levels: the present state of knowledge toward accomplishing pan-Pacific stratigraphic correlation. In Proceedings of the First International Congress on Pacific Neogene Stratigraphy. (International Union of Geological Sciences Commission on Stratigraphy Regional Committee on Pacific Neogene Stratigraphy, Science Council of Japan, Geological Society of Japan), 61–80.
Saito, T., Thompson, P.R., and Breger, D., 1976. Skeletal ultramicrostructure of some elongate-chambered planktonic foraminifera and related species. In Takayanagi, Y. and Saito, T., Progress in Micropaleontology: Selected Papers in Honor of Prof. Kiyoshi Asano. New York (Micropaleontology Press), 278–304.
Saito, T., Thompson, P.R., and Breger, D., 1981. Systematic index of Recent and Pleistocene planktonic foraminifera: Tokyo (University of Tokyo Press).
Schiebel, R., and Hemleben, C., 2017. Planktic Foraminifers in the Modern Ocean: Berlin (Springer). https://doi.org/10.1007/978-3-662-50297-6
Schlitzer, R., 2022. Ocean Data View. https://odv.awi.de
Schmuker, B., and Schiebel, R., 2002. Planktic foraminifers and hydrography of the eastern and northern Caribbean Sea. Marine Micropaleontology, 46(3):387–403. https://doi.org/10.1016/S0377-8398(02)00082-8
Schneider, C.E., and Kennett, J.P., 1996. Isotopic evidence for interspecies habitat differences during evolution of the Neogene planktonic foraminiferal clade Globoconella. Paleobiology, 22(2):282–303. https://doi.org/10.1017/S0094837300016225
Schubert, R.J., 1910. Über Foraminiferen und einen Fischotolithen aus dem fossilen Globigerinenschlamm von Neu-Guinea. Verhandlungen der kaiserlich-königlichen geologischen Reichsanstalt, 14:318–328.
Schwager, C., 1866. Fossile Foraminiferen von Kar Nikobar. Reise der Österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Urbair, 2(2):187–268. http://www.mdz-nbn-resolving.de/urn/resolver.pl?urn=urn:nbn:de:bvb:12-bsb10226528-7
Scott, G.H., 1979. The late Miocene to early Pliocene history of the Globorotalia miozea plexus from Blind River, New Zealand. Marine Micropaleontology, 4:341–361. https://doi.org/10.1016/0377-8398(79)90024-0
Scott, G.H., 1980. Globorotalia inflata lineage and G. crassaformis from Blind River, New Zealand: recognition, relationship, and use in uppermost Miocene-lower Pliocene biostratigraphy. New Zealand Journal of Geology and Geophysics, 23(5-6):665–677. https://doi.org/10.1080/00288306.1980.10424137
Scott, G.H., Bishop, S., and Burt, B.J., 1986. Guide to some Neogene Globorotalids (Foraminiferida) from New Zealand, New Zealand Geological Survey Paleontological Bulletin 61, Lower Hutt, NZ: (New Zealand Geological Survey).
Scott, G.H., Bishop, S., and Burt, B.J., 1990. Guide to some Neogene Globorotalids (Foraminiferida) from New Zealand. New Zealand Geological Survey Paleontological Bulletin, 6:1–135.
Scott, G.H., Kennett, J.P., Wilson, K.J., and Hayward, B.W., 2007. Globorotalia puncticulata: population divergence, dispersal and extinction related to Pliocene–Quaternary water masses. Marine Micropaleontology, 62(4):235–253. https://doi.org/10.1016/j.marmicro.2006.08.007
Seears, H.A., Darling, K.F., and Wade, C.M., 2012. Ecological partitioning and diversity in tropical planktonic foraminifera. BMC Evolutionary Biology, 12. https://doi.org/10.1186/1471-2148-12-54
Seiglie, G.A., 1963. Una nueva especie del genero Globigerina del Reciente de Venezuela. Boletín del Instituto Oceanográfico, Oriente Univ., Cumana Venezuela, 2(1):90–91.
Setty, M.G.A.P., 1977. Occurrence of Neogloboquadrina pachyderma new subspecies in the shelf-slope sediments of Northern Indian Ocean. Indian Journal of Marine Sciences, 6:72–75. http://drs.nio.org/drs/handle/2264/5434
Shackleton, N.J., and Vincent, E., 1978. Oxygen and carbon isotope studies in recent foraminifera from the southwest Indian ocean. Marine Micropaleontology, 3(1):1–13. https://doi.org/10.1016/0377-8398(78)90008-7
Shrivastav, A., Singh, A.K., Sinha, D.K., Kaushik, T., Singh, V.P., and Mallick, K, 2016. Significance of Globigerina bulloides d'Orbigny: a foraminiferal proxy for palaeomonsoon and past upwelling records. Journal of Climate Change, 2(2):99–110. https://doi.org/10.3233/JCC-160021
Simon, M.H., Arthur, K.L., Hall, I.R., Peeters, F.J.C., Loveday, B.R., Barker, S., Ziegler, M., and Zahn, R., 2013. Millennial-scale Agulhas Current variability and its implications for salt-leakage through the Indian–Atlantic Ocean Gateway. Earth and Planetary Science Letters, 383:101–112. https://doi.org/10.1016/j.epsl.2013.09.035
Singh, A.K., and Sinha, D.K., 2022. Plio-Pleistocene planktic foraminiferal biochronology of ODP Site 762B, Exmouth Plateau, Southeast Indian Ocean. Journal of Foraminiferal Research, 52(4):248–263. https://doi.org/10.2113/gsjfr.52.4.248
Singh, A.K., Sinha, D.K., Mallick, K., Singh, V.P., and Shrivastava, A., 2021. Diachronism in Late Neogene-Quaternary planktic foraminiferal events in Northern and Eastern Indian Ocean: Palaeoceanographic implications. Journal of the Palaeontological Society of India, 66(2):357–374. https://doi.org/10.1177/0971102320210219
Singh, V.P., Dwivedi, R., and Pathak, S., 2025. Late Neogene-Quaternary planktic foraminiferal biostratigraphy of IODP Hole U1474A, Agulhas Current region, Southwest Indian Ocean. Journal of Foraminiferal Research, 55(4):375–396. https://doi.org/10.61551/gsjfr.55.4.375
Singh, V.P., Pathak, S., and Dwivedi, R., 2023. Reduction in the strength of Agulhas Current during Quaternary: planktic foraminiferal records for 1.2 million years from IODP Hole U-1474A. Journal of Climate Change, 9:45–52. https://doi.org/10.3233/JCC230031
Sinha, D.K., and Singh, A.K., 2008. Late Neogene planktic foraminiferal biochronology of the ODP Site 763A, Exmouth Plateau, Southeast Indian Ocean. Journal of Foraminiferal Research, 38(3):251–270. https://doi.org/10.2113/gsjfr.38.3.251
Sinha, D.K., Singh, A.K., and Tiwari, M., 2006. Palaeoceanographic and palaeoclimatic history of ODP site 763A (Exmouth Plateau), southeast Indian Ocean: 2.2 Ma record of planktic foraminifera. Current Science, 90(10):1363-1369. https://www.jstor.org/stable/24091985
Spezzaferri, S., 1994. Planktonic foraminiferal biostratigraphy and taxonomy of the Oligocene and lower Miocene in the oceanic record: an overview. Palaeontographica Italica, 81:1–187.
Spezzaferri, S., Coxall, H.K., Olsson, R.K., and Hemleben, C., 2018. Taxonomy, biostratigraphy, and phylogeny of Oligocene Globigerina, Globigerinella, and Quiltyella n. gen. In Wade, B.S., Olsson, R.K., Pearson, P.N., Huber, B.T. and Berggren, W.A., Atlas of Oligocene planktonic Foraminifera. Cushman Foundation Special Publication, 46: (Cushman Foundation for Foraminiferal Research), 179–214. https://pubs.geoscienceworld.org/cushmanfoundation/books/edited-volume/2271/chapter-abstract/126275365/TAXONOMY-BIOSTRATIGRAPHY-AND-PHYLOGENY-OF
Spezzaferri, S., Kucera, M., Pearson, P.N., Wade, B.S., Rappo, S., Poole, C.R., Morard, R., and Stalder, C., 2015. Fossil and genetic evidence for the polyphyletic nature of the planktonic foraminifera "Globigerinoides", and description of the new genus Trilobatus. PloS One, 10(5):e0128108. https://doi.org/10.1371/journal.pone.0128108
Spezzaferri, S., and Premoli Silva, I., 1991. Oligocene planktonic foraminiferal biostratigraphy and paleoclimatic interpretation from Hole 538A, DSDP Leg 77, Gulf of Mexico. Palaeogeography, Palaeoclimatology, Palaeoecology, 83(1):217–263. https://doi.org/10.1016/0031-0182(91)90080-B
Srinivasan, M.S., and Kennett, J.P., 1976. Evolution and phenotypic variation in the late Cenozoic Neogloboquadrina dutertrei plexus. In Takayanagi, Y., and Saito, T. (Eds.), Progress in Micropaleontology: Selected Papers in Honor of Prof. Kiyoshi Asano. New York (Micropaleontology Press), 329–355.
Srinivasan, M.S., and Kennett, J.P., 1981a. Neogene planktonic foraminiferal biostratigraphy and evolution: Equatorial to subantarctic, South Pacific. Marine Micropaleontology, 6(5):499–533. https://doi.org/10.1016/0377-8398(81)90019-0
Srinivasan, M.S., and Kennett, J.P., 1981b. A review of Neogene planktonic foraminiferal biostratigraphy: applications in the Equatorial and South Pacific. In Warme, J.E., Douglas, R.G. and Winterer, E.L., The Deep Sea Drilling Project: A Decade of Progress. 32: (SEPM Society for Sedimentary Geology). https://doi.org/10.2110/pec.81.32.0395
Srinivasan, M.S., and Sinha, D.K., 1991. Improved correlation of the late Neogene planktonic foraminiferal datums in the equatorial to cool subtropical DSDP Sites, Southwest Pacific: application of the graphic correlation method. Geological Society of India Memoir, 20:55–93.
Srinivasan, M.S., and Sinha, D.K., 1992. Late Neogene planktonic foraminiferal events of the southwest Pacific and Indian Ocean – a comparison. In Tsuchi, R., and Ingle, J.C., Pacific Neogene: Environment, Evolution, and Events. Tokyo (University of Tokyo Press), 203–220.
Srinivasan, M.S., and Sinha, D.K., 2000. Ocean circulation in the tropical Indo-Pacific during early Pliocene (5.6-4.2 Ma): paleobiogeographic and isotopic evidence. Journal of Earth System Science, 109(3):315–328. https://doi.org/10.1007/BF03549815
Stainforth, R.M., Lamb, J. L., Luterbache, H., Beard, J. H. and Jeffords, R.M., 1975. Cenozoic Planktonic Foraminiferal Zonation and Characteristics of Index Forms: (The Paleontological Institute, The University of Kansas). https://hdl.handle.net/1808/3836
Steineck, P.L., and Fleisher, R.L., 1978. Towards the classical evolutionary reclassification of Cenozoic Globigerinacea (Foraminiferida). Journal of Paleontology, 52(3):618–635. http://www.jstor.org/stable/1303967
Subbotina, N.N., 1958. New genera and species of foraminifera. In Bykova, N.K., Microfauna of the USSR–Part IX. Trudy Vsesoyuznego Neftyanogo Nauchno-Issledovatel'skogo Geologo-Razvedochnogo Instituta (VNIGRI), 115: 58–61.
Takayanagi, Y., and Saito, T., 1962. Planktonic foraminifera from the Nobori formation, Shikoku, Japan. Science Reports of the Tohoku University Second Series (Geology), 2(5):67–105.
Theyer, F., 1973. Globorotalia inflata triangula, a new planktonic foraminiferal subspecies. Journal of Foraminiferal Research, 3(4):199–201. https://doi.org/10.2113/gsjfr.3.4.199
Thiede, J., 1975. Distribution of foraminifera in surface waters of a coastal upwelling area. Nature, 253(5494):712–714. https://doi.org/10.1038/253712a0
Thiede, J., 1983. Skeletal plankton and nekton in upwelling water masses off northwestern South America and Northwest Africa. In Suess, E., and Thiede, J. (Eds.), Coastal Upwelling: Its Sediment Record (Part A): Response of the Sedimentary Regime to Present Coastal Upwelling: New York (Plenum), 183–207.
Thompson, P.R., 1973. Two new Late Pleistocene planktonic foraminifera from a core in the Southwest Indian Ocean. Micropaleontology, 19(4):469–474. http://www.jstor.org/stable/1484909
Thunell, R.C., 1978. Distribution of recent planktonic foraminifera in surface sediments of the Mediterranean Sea. Marine Micropaleontology, 3(2):147–173. https://doi.org/10.1016/0377-8398(78)90003-8
Thunell, R.C., and Sautter, L.R., 1992. Planktonic foraminiferal faunal and stable isotopic indices of upwelling: a sediment trap study in the San Pedro Basin, Southern California Bight. Geological Society, London, Special Publications, 64(1):77–91. https://doi.org/10.1144/GSL.SP.1992.064.01.05
Todd, R., 1957. Smaller foraminifera. In Geology of Saipan, Mariana Islands. Part 3, Paleontology. US Geological Survey Professional Paper, 280–E–J: Washington (US Government Printing Office), 265–320. https://pubs.usgs.gov/pp/0280e-j/report.pdf
Van den Broeck, E., 1876. Etude sur les Foraminifères de la Barbade (Antilles). Annales de la Société belge de microscopie: Mémoires, 2:55–152.
Vincent, E., and Toumarkine, M., 1990. Neogene planktonic foraminifers from the western tropical Indian Ocean, Leg 115. In Duncan, R.A., Backman, J., Peterson, L.C., et al., Proceedings of the Ocean Drilling Program, Scientific Results, 115: College Station, TX (Ocean Drilling Program), 795–836. https://doi.org/10.2973/odp.proc.sr.115.143.1990
Wade, B.S., Olsson, R.K., Pearson, P.N., Huber, B.T., and Berggren, W.A., 2018. Atlas of Oligocene Planktonic Foraminifera. Special Publication - Cushman Foundation for Foraminiferal Research, 46.
Wade, B.S., Pearson, P.N., Berggren, W.A., and Pälike, H., 2011. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth-Science Reviews, 104(1–3):111–142. https://doi.org/10.1016/j.earscirev.2010.09.003
Walters, R., 1965. The Globorotalia zealandica and G. miozea lineages. New Zealand Journal of Geology and Geophysics, 8(1):109–127. https://doi.org/10.1080/00288306.1965.10422135
Wang, L., 2000. Isotopic signals in two morphotypes of Globigerinoides ruber (white) from the South China Sea: implications for monsoon climate change during the last glacial cycle. Palaeogeography, Palaeoclimatology, Palaeoecology, 161(3):381–394. https://doi.org/10.1016/S0031-0182(00)00094-8