Betzler, C., Eberli, G.P., Alvarez Zarikian, C.A., and the Expedition 359 Scientists
Proceedings of the International Ocean Discovery Program Volume 359
publications.iodp.org
doi:10.14379/iodp.proc.359.111.2017
Data report: surface seawater plankton sampling for coccolithophores undertaken during IODP Expedition 3591
Jeremy R. Young, Santi Pratiwi, Xiang Su, and the Expedition 359 Scientists2
Keywords: International Ocean Discovery Program, IODP, JOIDES Resolution, Expedition 359, Indian Ocean transect, Maldives, coccolithophores
MS 359-111: Published 4 May 2017
Abstract
Data on extant coccolithophore assemblages from plankton samples collected during International Ocean Discovery Program Expedition 359 to the Maldives is presented. Samples include 12 collected during passage across the Indian Ocean from Darwin, Australia, to the Maldives and 40 collected in the Maldives. Assemblages were analyzed by light and scanning electron microscopy, and detailed assemblage data are presented. Comparison with previous data from the region suggests that there are consistent distinctive aspects to Indian Ocean assemblages.
Introduction
Knowledge of coccolithophore biogeography is an essential basis for paleoecological interpretation of coccolithophore assemblages and for investigation of evolutionary patterns (e.g., Winter et al., 1994; Knappertsbusch et al., 1997; Baumann et al., 2005). However, there have been relatively few large-scale surveys of coccolithophores, and their biogeography is poorly known in many areas, notably the Indian Ocean. There have been some useful studies of Indian Ocean coccolithophores, including a transect from Java to Aden (Kleijne et al., 1989; Kleijne, 1993), a study of the southeast Indian Ocean (Takahashi and Okada 2000), studies of coccolithophores in various upwelling areas (Andruleit et al., 2003, 2004, 2005; Schiebel et al., 2004; Andruleit, 2007), and a short north–south transect study in the equatorial Indian Ocean (Guptha et al., 2005). However, this sampling has been patchy, there has been little resampling of areas studied, and the data have not been synthesized. Consequently, the coccolithophore assemblages of the Indian Ocean are poorly characterized, and in particular it is difficult to determine how they differ from those in the Atlantic and Pacific Oceans, even though it is becoming increasingly clear that there are significant differences between Pacific and Atlantic Ocean assemblages (Hagino and Young, 2015).
International Ocean Discovery Program (IODP) Expedition 359 commenced with a transit across the equatorial Indian Ocean from Darwin, Australia, to the Maldives (Figure F1A). This transit presented a valuable opportunity to sample the equatorial assemblages to determine broad patterns of coccolithophore distribution and compare them with those recorded previously, notably by Kleijne et al. (1989). Sampling continued within the Maldives drilling area to (1) determine whether assemblages within the Maldives show evidence of ecological restriction or modified assemblages related to the particular environment of the atoll chain, (2) investigate reproducibility of assemblage data by repeat sampling within a limited area over an extended period, and (3) investigate the potential of the Maldives for coccolithophore research.
This report provides a concise overview of the sampling undertaken and the preliminary results. Fuller documentation and analysis will be published later, in conjunction with other data.
Materials and methods
Seawater samples for plankton study were collected in two ways. While the ship was under way, samples were collected from the sea surface using a bucket and rope from the starboard main deck. While the ship was stationary at drill sites, a plankton sampling bottle (WildCo Beta 8.3 L van Dorn style in situ sample bottle, shipboard equipment) was deployed to collect water from ~15 m below the water’s surface. Using a plankton sampling bottle to sample from depth is preferable for obtaining cleaner samples and to avoid the possibility of sampling water from which the plankton has settled out, but in practice, both sampling techniques yielded good assemblages. After sampling, 1 or 2 L of seawater were vacuum filtered onto microfilter discs using 25 mm filtration funnels (Pall Laboratory #4203) on a stainless steel filter ramp (shipboard equipment). Prefiltration through a brass 38 µm test sieve was used to remove larger zooplankton and contaminants (this was more important for the bucket-collected samples). Two filter disc types were used: 0.8 µm pore cellulosic filters (Sartorius Stedim Cellulose Nitrate) and 0.8 µm pore polycarbonate track-etched filters (Whatman Nuclepore). After collection, the filters were oven dried (at 40°–60°C) and stored in 47 mm plastic petrislides (Millipore). For light microscopy, a portion of cellulosic filter was cut out and mounted on a microscope slide using Norland optical adhesive (NOA) 74, a low-viscosity version of the standard NOA61 adhesive that penetrates better into the filter mesh. For electron microscopy, portions of the polycarbonate filters were cut out and mounted on aluminum stubs using carbon tabs and sputter coated with gold-palladium using a Leitz EM ACE2000 sputter coater.
Samples were examined and assemblages counted at 1000× magnification using a Zeiss Axioplan microscope with cross-polarized illumination (Tables T1, T2). About 150 coccospheres were counted and identified, mostly to species level. For taxonomy, the syntheses of Young et al. (2003, 2014) and Jordan et al. (2004) were followed. Counts were converted into cell densities per liter by recording the area counted and calculating the proportion of the filter surface this represented. Selected samples were examined by electron microscopy using the shipboard Hitachi TM3000 scanning electron microscope (SEM) to confirm identification and identify smaller species that cannot be recognized using light microscopy (Table T3).
T1. Assemblage data for the Indian Ocean transect. Download table in .csv format.
T2. Assemblage data for the Maldives sites. Download table in .csv format.
T3. Scanning electron microscopy occurrence data. Download table in .csv format.
Results
Indian Ocean transect
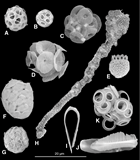
Thirteen surface water samples (Plkt-1 to Plkt-13) were collected during the passage across the Indian Ocean from Darwin, Australia, to the Maldives (Figure F1A). The assemblages counted by light microscopy in these samples are listed in Table T1. The assemblage data are illustrated as cell concentrations of the principal taxa (Figure F1B) and as percentage abundance (Figure F1C) along the route. All samples were also examined by scanning electron microscopy, and the species observed are listed in Table T3, although it should be noted that the time spent per sample was highly variable. Figure F2 illustrates several of the most common species.
The data can be divided into three parts. There are low abundance assemblages (<15,000 coccospheres/L) at the beginning and end of the transect separated by higher abundance samples (>25,000 coccospheres/L) from two stations, Plkt-6 and Plkt-7 (Figure F1B).
The first five samples (Plkt-1 to Plkt-5), from the eastern part of the transect, are characterized by high relative abundances of Umbellosphaera irregularis and Emiliania huxleyi with subsidiary Rhabdosphaera clavigera var. stylifer, Discosphaera tubifera, and Gephyrocapsa oceanica.
The next two samples (Plkt-6 and Plkt-7), from southwest of Java, have higher abundances (>25,000 coccospheres/L) and are characterized by high relative abundances of E. huxleyi, G. oceanica, and Oolithotus antillarum. Algirosphaera robusta and Umbilicosphaera sibogae are also notably common. Umbellosphaera and Rhabdosphaera are almost absent from these two samples.
The final six samples (Plkt-8 to Plkt-13) again have low abundances (<15,000 coccospheres/L) and are dominated by G. oceanica, U. irregularis, and Umbilicosphaera hulburtiana. D. tubifera is also common, and Michaelsarsia adriaticus noticeably abundant in one sample (Plkt-9). Conversely, E. huxleyi is unusually rare, and R. clavigera is absent.
Maldives assemblages
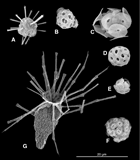
Forty water samples (Plkt-14 to Plkt-53) were collected, approximately daily, during the drilling operations within the Maldives. Water temperature and salinity were measured on several occasions, and they remained constant with salinities of 35 and temperatures of 27°–28°C. The assemblages counted by light microscopy in these samples are listed in Table T2. The assemblage data are illustrated as cell concentrations of the principal taxa (Figure F2A) and as percentage abundance (Figure F2B) along the route. Several samples were also observed by scanning electron microscopy, and all species observed were imaged as summarized in Table T3. Figure F3 illustrates several of the most common species.
All assemblages have low-abundance coccolithophore populations, with between 4,000 and 12,000 cells/L, and in all samples the dominant species is Gephyrocapsa. The rest of the assemblage is also broadly constant throughout the sampling period. The second and third most abundant species are usually U. irregularis and D. tubifera, although this ranking becomes more variable in the last six samples counted (Plkt-36 to Plkt-41) (Table T2). Other consistently present species are Ceratolithus cristatus (heterococcolith and ceratolith phase), E. huxleyi (all specimens seen in the SEM were Type A), Helicosphaera hyalina, Coronosphaera mediterranea, Michaelsarsia (all specimens seen in SEM were Michaelsarsia adriatica), Umbellosphaera tenuis (all specimens seen in the SEM were Type I), Poricalyptra magnaghii (identified in all SEM samples examined), Syracosphaera exigua (identified in all SEM samples examined), and Syracosphaera halldallii (identified in all SEM samples examined).
Superimposed on this pattern of general uniformity is some apparently random variability and some evidence of change through time. Random variability is most obviously seen in Gephyrocapsa abundance and in total cell numbers (Figure F4; Table T2). Samples from the sites at the mouth of the Kardiva Channel (Sites U1465, U1467, and U1469) showed more variability than those further into the Inner Sea (Sites U1467 and U1468).
Apparent secular change through the sampling interval includes a general increase in the proportion of G. oceanica accompanied by appearance and increase in abundance of E. huxleyi. In parallel, U. irregularis and D. tubifera decline in abundance.
One of the most conspicuous aspects of the assemblages is the consistent presence of Ceratolithus in multiple phases. This includes loose ceratoliths, ceratoliths surrounded by hoop coccoliths, numerous loose heterococcoliths, and frequent coccospheres of the heterococcolith phase. The heterococcolith coccospheres often include hoop coccoliths and coccoliths intermediate in morphology between fully formed heterococcoliths and hoop coccoliths. All observed ceratoliths were of the Ceratolithus cristatus var. telesmus type, and all complete heterococcoliths were of the Ceratolithus cristatus var. coccolithomorpha type. Ceratolithus was evidently thriving in this environment during the sampling. Ceratoliths were, however, rare in the “mudline” Holocene samples collected during the expedition and virtually absent in the Pleistocene and Pliocene sediments.
Biogeography
The basic pattern shown in the transect data of an upwelling community dominated by placolith coccoliths (Emiliania, Gephyrocapsa, and Umbilicosphaera) contrasted with oligotrophic assemblages dominated by Umbellosphaera and Discosphaera is exactly as has often been described (e.g., Young, 1994; Winter et al., 1994). The details, however, are distinctive and are reinforced by the results from the Maldives. These include
- The dominant Umbellosphaera species is U. irregularis, and U. tenuis, when present, is represented by the lightly calcified but ornamented Type I of Kleijne (1993). This classification applies to all 40 samples studied.
- Rhabdosphaera stylifera is common in the southeast Indian Ocean but absent from the rest of the transect and all the Maldives samples.
- E. huxleyi is present in the southeast Indian Ocean and in the Java upwelling but absent from the rest of the transect. E. huxleyi did appear in the Maldives in the later part of the survey, but all specimens are Type A, whereas in the equatorial upwelling they are primarily Type C.
- Gephyrocapsa ericsonii and Reticulofenestra parvula are notably absent.
- S. exigua, which is only very sporadically recorded elsewhere, is a common member of these assemblages, especially in the Maldives. Other unusual species encountered repeatedly include P. magnaghii (present in nearly every sample examined by SEM, but no other Poricalyptra species were observed), Calicasphaera diconstricta (a species which has almost never been recorded since its original description by Kleijne [1993]), and Poritectolithus maximus.
- Ceratolithus assemblages are exclusively composed of the telesmus type ceratoliths and coccolithomorpha type heterococcoliths.
These aspects have not previously been mentioned as features of Indian Ocean assemblages, but intriguingly, comparison with the available literature suggests that they are all consistent features. The most relevant comparisons are with Kleijne (1993) for the northern Indian Ocean and with Takahashi and Okada (2000) for the southeast Indian Ocean. Kleijne (1993) recognized four assemblages in the northern Indian:
- Ocean-A-1: E. huxleyi dominated with G. oceanica,
- B: G. oceanica dominated,
- C-1: U. irregularis dominated, and
- D: Syracosphaeraceae and G. oceanica dominated.
Our assemblages could mostly be characterized as a mixture of the B and C-1 groups of Kleijne (1993). This includes both the dominant species, G. oceanica, U. irregularis, and U. tenuis Type I and the more distinctive minor species C. cristatus var. telesmus, S. exigua, and P. magnaghii. It is also noticeable that R. clavigera, normally an almost ubiquitous species in oligotrophic warm-water assemblages, is absent. Similarly, the assemblages recorded from the southeast Indian Ocean by Takahashi and Okada (2000) are similar to those we encountered in that area, with an assemblage dominated by U. irregularis and E. huxleyi with common D. tubifera and R. clavigera.
Overall, there is intriguing evidence that the Indian Ocean equatorial and subtropical coccolithophore assemblages have stable characteristic features that distinguish them from the equivalent assemblages in both the Atlantic and Pacific Oceans. Further characterization of these patterns and of the interplay of ecological and molecular genetic controls causing them would be useful to study.
The assemblages from the Maldives are broadly similar to those from the open waters of the north Indian Ocean and the Arabian Sea as sampled during this expedition and recorded in previous studies. The most distinctive element of the assemblage was the high abundance of Ceratolithus throughout the sampling period. However, this abundance was not reflected in the surface sediment samples, and it may have been an unusual event. Distinctive inner neritic elements such as Cruciplacolithus neohelis, Pleurochrysis, Braarudosphaera, or Tergestiella were not observed, but it was not possible to sample from shallow water or lagoonal locations. Overall, the sampling was very productive in terms of recovery of diverse assemblages, and this clearly would be a good field area for coccolithophore research.
See the Appendix for a list of all taxa recorded.
Acknowledgments
We are grateful for much support from the crew of the R/V JOIDES Resolution, IODP staff, and shipboard science party with this study. Most especially, we thank Lisa Crowder and Douglas Cummings for their help with the plankton sampling. Kaoro Niino assisted with water filtering, and Or Bialik helped with diagram drafting. We thank Denise Kulhanek for a careful review of the original manuscript.
References
Andruleit, H., 2007. Status of the Java upwelling area (Indian Ocean) during the oligotrophic Northern Hemisphere winter monsoon season as revealed by coccolithophores. Marine Micropaleontology, 64(1–2):36–51. http://dx.doi.org/10.1016/j.marmicro.2007.02.001
Andruleit, H., Rogalla, U., and Stäger, S., 2004. From living communities to fossil assemblages: origin and fate of coccolithophores in the northern Arabian Sea. Micropaleontology, 50(Supplement 1):5–21. http://dx.doi.org/10.2113/50.Suppl_1.5
Andruleit, H., Rogalla, U., and Stäger, S., 2005. Living coccolithophores recorded during the onset of upwelling conditions off Oman in the western Arabian Sea. Journal of Nannoplankton Research, 27(1):1–14. http://ina.tmsoc.org/JNR/online/27/Andruleit et al JNR 27-1.pdf
Andruleit, H., Stäger, S., Rogalla, U., and Cepek, P., 2003. Living coccolithophores in the northern Arabian Sea: ecological tolerances and environmental control. Marine Micropaleontology, 49(1–2):157–181. http://dx.doi.org/10.1016/S0377-8398(03)00049-5
Baumann, K.-H., Andruleit, H., Böckel, B., Geisen, M., and Kinkel, H., 2005. The significance of extant coccolithophores as indicators of ocean water masses, surface water temperature, and paleoproductivity: a review. Paläeontologische Zeitschrift, 79(1):93–112. http://dx.doi.org/10.1007/BF03021756
Guptha, M.V.S., Mergulhao, L.P., Murty, V.S.N., and Shenoy, D.M., 2005. Living coccolithophores during the northeast monsoon from the equatorial Indian Ocean: implications on hydrography. Deep Sea Research, Part II: Topical Studies in Oceanography, 52(14–15):2048–2062. http://dx.doi.org/10.1016/j.dsr2.2005.05.010
Hagino, K., and Young, J.R., 2015. Biology and paleontology of coccolithophores (Haptophytes). In Ohtsuka, S., Suzaki, T., Horiguchi, T., Suzuki, N., and Not, F. (Eds.), Marine Protists: Diversity and Dynamics: Tokyo (Springer), 311–330. http://dx.doi.org/10.1007/978-4-431-55130-0_12
Jordan, R.W., Cros, L., and Young, J.R., 2004. A revised classification scheme for living Haptophytes. Micropaleontology, 50(Supplement 1):55–79. http://dx.doi.org/10.2113/50.Suppl_1.55
Kleijne, A., 1993. Morphology, Taxonomy and Distribution of Extant Coccolithophorids (Calcareous Nannoplankton): Katwijk, The Netherlands (Drukkerij FEBO B.V.).
Kleijne, A., Kroon, D., and Zevenboom, W., 1989. Phytoplankton and foraminiferal frequencies in northern Indian Ocean and Red Sea surface waters. Netherlands Journal of Sea Research, 24(4):531–539. http://dx.doi.org/10.1016/0077-7579(89)90131-2
Knappertsbusch, M., Cortes, M.Y., and Thierstein, H.R., 1997. Morphologic variability of the coccolithophorid Calcidiscus leptoporus in the plankton, surface sediments and from the early Pleistocene. Marine Micropaleontology, 30(4):293–317. http://dx.doi.org/10.1016/S0377-8398(96)00053-9
Schiebel, R., Zeltner, A., Treppke, U.F., Waniek, J.J., Bollmann, J., Rixen, T., and Hemleben, C., 2004. Distribution of diatoms, coccolithophores and planktic foraminifers along a trophic gradient during SW monsoon in the Arabian Sea. Marine Micropaleontology, 51(3–4):345–371. http://dx.doi.org/10.1016/j.marmicro.2004.02.001
Takahashi, K., and Okada, H., 2000. Environmental control on the biogeography of modern coccolithophores in the southeastern Indian Ocean offshore of Western Australia. Marine Micropaleontology, 39(1–4):73–86. http://dx.doi.org/10.1016/S0377-8398(00)00015-3
Winter, A., Jordan, R.W., and Roth, P.H., 1994. Biogeography of living coccolithophores in ocean waters. In Winter, A. and Siesser, W.G. (Eds.), Coccolithophores. Cambridge, United Kingdom (Cambridge University Press), 161–177.
Young, J.R., 1994. Functions of coccoliths. In Winter, A., and Siesser, W.G. (Eds.), Coccolithophores: Cambridge, United Kingdom (Cambridge University Press), 63–82.
Young, J.R., Bown, P.R., and Lees, J.A. (Eds.). Nannotax3. http://ina.tmsoc.org/Nannotax3 (accessed 24 September 2014).
Young, J.R., Geisen, M., Gros, L., Kleyne, A., Sprengel, C., Probert, I., and Ostergard, J., 2003. A guide to extant coccolithophore taxonomy. Journal of Nannoplankton Research, 1:1–125. http://ina.tmsoc.org/JNR/online/SpecIssue/Young_et_al_2003_JNR Special_Issue_TaxonomyGuide.pdf
Appendix
Full citations are given here for all taxa recorded. Bibliographic references can be found in Young et al. (2003) or on the Nannotax website (Young et al., 2014).
- Acanthoica quattrospina Lohmann, 1903
- Algirosphaera robusta (Lohmann, 1902) Norris, 1984
- Alisphaera gaudii Kleijne et al., 2002
- Alisphaera unicornis Okada and McIntyre, 1977
- Anthosphaera fragaria Kamptner, 1937
- Calcidiscus leptoporus subsp. quadriperforatus (Kamptner, 1937) Geisen et al., 2002 HOL
- Calciosolenia murrayi Gran, 1912
- Calciopappus rigidus Heimdal in Heimdal and Gaarder, 1981
- Calicasphaera diconstricta Kleijne, 1991
- Calyptrolithina multipora (Gaarder in Heimdal and Gaarder, 1980) Norris, 1985
- Calyptrosphaera galea Lecal-Schlauder, 1951
- Calyptrosphaera sphaeroidea Schiller, 1913
- Ceratolithus cristatus Norris, 1965 CER telesmus type, sensu Young et al., 2003
- Ceratolithus cristatus Norris, 1965 HET coccolithomorpha type, sensu Young et al., 2003
- Ceratolithus cristatus Norris, 1965 HET hoops type, sensu Young et al., 2003
- Corisphaera gracilis Kamptner, 1937
- Corisphaera sp.
- Coronosphaera mediterranea (Lohmann, 1902) Gaarder in Gaarder and Heimdal, 1977
- Cyrtosphaera aculeata (Kamptner, 1941) Kleijne, 1992
- Discosphaera tubifera (Murray and Blackman, 1898) Ostenfeld, 1900
- Emiliania huxleyi (Lohmann, 1902) Hay and Mohler in Hay et al., 1967 Type B/C
- Emiliania huxleyi (Lohmann, 1902) Hay and Mohler in Hay et al., 1967 Type A
- Gephyrocapsa oceanica Kamptner, 1943
- Helicosphaera hyalina Gaarder, 1970
- Helicosphaera carteri (Wallich, 1877) Kamptner, 1954
- Helladosphaera cornifera (Schiller, 1913) Kamptner, 1937
- Helladosphaera pienaarii Norris, 1985
- Homozygosphaera arethusae (Kamptner, 1941) Kleijne, 1991 HOL
- Homozygosphaera spinosa (Kamptner, 1941) Deflandre, 1952
- Homozygosphaera triarcha Halldal and Markali, 1955
- Homozygosphaera vercellii Borsetti and Cati, 1979
- Michaelsarsia adriaticus (Schiller, 1914) Manton et al., 1984
- Oolithotus antillarum (Cohen, 1964) Reinhardt in Cohen and Reinhardt, 1968
- Ophiaster hydroideus (Lohmann, 1903) Lohmann, 1913
- Palusphaera vandelii Lecal, 1965
- Polycrater galapagensis Manton and Oates, 1980
- Poricalyptra magnaghii (Borsetti and Cati, 1976) Kleijne, 1991
- Poritectolithus maximus Kleijne, 1991
- Rhabdosphaera clavigera var. stylifera (Lohmann, 1902) Kleijne and Jordan, 1990
- Rhabdosphaera xiphos (Deflandre and Fert, 1954) Norris, 1984
- Scyphosphaera apsteinii Lohmann, 1902
- Syracosphaera bannockii HOL (Borsetti and Cati, 1976) Cros et al., 2000
- Syracosphaera corolla Lecal, 1966
- Syracosphaera exigua Okada and McIntyre, 1977
- Syracosphaera halldalii Gaarder in Gaarder and Hasle, 1971 ex Jordan, 1994
- Syracosphaera halldalii Gaarder in Gaarder and Hasle 1971, ex Jordan, 1994 HOL
- Syracosphaera hastata Kleijne and Cros, 2009
- Syracosphaera histrica Kamptner, 1941 HOL
- Syracosphaera leptolepis Kleijne and Cros, 2009
- Syracosphaera molischii Schiller, 1925
- Syracosphaera nana (Kamptner, 1941) Okada and McIntyre, 1977
- Syracosphaera nodosa Kamptner, 1941
- Syracosphaera noroitica Knappertsbusch, 1993
- Syracosphaera orbiculus Okada and McIntyre, 1977
- Syracosphaera ossa (Lecal, 1966) Loeblich and Tappan, 1968
- Syracosphaera prolongata Gran, 1912 ex Lohmann, 1913
- Syracosphaera pulchra Lohmann, 1902
- Syracosphaera pulchra Lohmann, 1902 HOL oblonga type
- Syracosphaera rotula Okada and McIntyre, 1977
- Umbellosphaera irregularis Paasche in Markali and Paasche, 1955
- Umbellosphaera tenuis (Kamptner, 1937) Paasche in Markali and Paasche, 1955 Type I
- Umbilicosphaera foliosa (Kamptner, 1963) Geisen in Sáez et al., 2003
- Umbilicosphaera hulburtiana Gaarder, 1970
- Umbilicosphaera sibogae (Weber-van Bosse, 1901) Gaarder, 1970
- Zygosphaera amoena Kamptner, 1937
1 Young, J.R., Pratiwi, S., Su, X., and the Expedition 359 Scientists, 2017. Data report: surface seawater plankton sampling for coccolithophores undertaken during IODP Expedition 359. In Betzler, C., Eberli, G.P., Alvarez Zarikian, C.A., and the Expedition 359 Scientists, Maldives Monsoon and Sea Level. Proceedings of the International Ocean Discovery Program, 359: College Station, TX (International Ocean Discovery Program). http://dx.doi.org/10.14379/iodp.proc.359.111.2017
2 Expedition 359 Scientists’ addresses.
This work is distributed under the Creative Commons Attribution 4.0 International (CC BY 4.0) license. 
- F1. Coccolithophore assemblages along the transect across the Indian Ocean between Darwin and the Maldives.
- F2. Coccolithophore assemblages sampled within the Maldives during drilling.
- F3. SEM images of common coccolithophores from samples collected within the Maldives.
- F4. SEM images of common coccolithophores from the transect across the Indian Ocean.