| IODP Proceedings Volume contents Search | ||

|
||
| Expedition reports Research results Supplementary material Drilling maps Expedition bibliography | ||
|
doi:10.2204/iodp.proc.311.102.2006 Interstitial water geochemistryThe majority of shipboard IW samples were obtained on 5 to 30 cm long whole-round cores that were collected according to the following scheme. Generally, routine samples were collected from one of the holes at a frequency of approximately four samples per core from the seafloor to the sulfate/methane interface (SMI), followed by a sampling resolution of three whole-round samples in the first core below the SMI, two whole-round samples per core to a depth of two cores below the bottom-simulating reflector (BSR), and one sample per core below that to the total depth of the hole. These whole-round samples were cut on the catwalk, capped, and taken to the laboratory for immediate processing. Samples were taken at higher resolution (three to four samples per section) in the upper 15 m of holes dedicated for microbiological sampling. During high-resolution sampling and when there were too many IW samples to process immediately, capped whole-round core sections were stored under a nitrogen atmosphere until they were squeezed, which occurred no later than 12 h after core retrieval. Gloves were used during sample processing. After extrusion from the core liner, the surface of each whole-round core sample was carefully scraped with a clean spatula to remove potential contamination from seawater and sediment smearing in the borehole. In APC cores, 1 cm from the outer diameter, top, and bottom faces was removed. In XCB cores, where borehole contamination is higher, as much as 90% of the sediment was removed from each whole-round sample. In rare cases, the entire whole-round sample had to be discarded. The remaining sediment (~50–300 cm3) was placed into a titanium squeezer, modified after the stainless steel squeezer of Manheim and Sayles (1974). In most cases, gauge pressures up to 20 MPa were applied using a laboratory hydraulic press to extract interstitial water. In a few very dry cores recovered from greater depths, gauge pressure up to a maximum of 30 MPa was applied. Interstitial water was passed through a prewashed Whatman number 1 filter fitted above a titanium screen, filtered through a 0.2 ÁM polysulfone disposable filter, and subsequently extruded into a prewashed (10% HCl) plastic syringe attached to the bottom of the squeezer assembly. In most cases, 15–40 cm3 of pore water was collected from each sample, which required squeezing the sediment for 20–40 min. IW subsamples were first collected in a 10 mL syringe and immediately analyzed for salinity, pH, sulfate, and alkalinity. Using the 10 mL syringe avoided air bubbles, minimized contamination of this fraction of the interstitial water by dissolved oxygen, and allowed for efficient and rapid subsampling during sediment squeezing. After collection of the first 10 mL, a 50 mL syringe was used to collect the remaining interstitial water. Collection of subsamples for shore-based analysesSubsamples were collected in glass vials and ampules for shore-based isotopic characterization of the interstitial water (oxygen and deuterium) and dissolved metabolites (e.g., dissolved inorganic and organic carbon, sulfate, and sulfide). In addition, subsamples were collected for analyses of dissolved volatile fatty acids (in glass vials and frozen), halogens, minor and trace metal constituents, and their isotopes (in acid-cleaned plastic containers). Shipboard interstitial water analysesIW samples were analyzed for routine shipboard measurements according to standard procedures (Gieskes et al., 1991). Salinity was measured as total dissolved solids using a Goldberg optical handheld refractometer. The pH was determined by ion-selective electrode. Alkalinity was determined by Gran titration with a Metrohm autotitrator. Sulfate (SO4) concentration was measured by manual dilution and manual injection into a Dionex DX-120 ion chromatograph immediately after IW collection. A subsample was treated with CdNO3 solution, which precipitated the sulfide as CdS for subsequent shore-based concentration and S isotope analyses; this treatment prevented the sulfide from oxidizing to sulfate. High-precision chloride concentrations were determined by Mohr titration using silver nitrate (AgNO3). Quantification was based on comparison with International Association of the Physical Sciences of the Ocean (IAPSO) standard seawater. The values were corrected for the presence of other halogens assuming seawater ratios, as detailed by Gieskes et al. (1991). Dissolved calcium was either measured by titration with ethylene-bis-(oxyethylenenitrilo)-tetra-acetic acid and corrected for interference by magnesium or by inductively coupled plasma–atomic emission spectroscopy (ICP-AES). Similarly, dissolved magnesium was obtained by titration with disodium ethylenediamine-tetra-acetate and corrected for Ca or measured by ICP-AES. The analytical procedures and calculations involved in the alkaline earth analyses are detailed in Gieskes et al. (1991). Dissolved phosphate (PO4) and ammonium (NH4) concentrations were determined by spectrophotometric methods using a Milton Roy Spectronic 301 spectrophotometer using cuvettes or a Milton Roy sample introduction system (Gieskes et al., 1991). Major and selected minor element concentrations were obtained by ICP-AES with a Jobin Yvon JY2000 spectrometer using dilutions of IAPSO standard seawater as calibration standards. For calcium, magnesium, sodium, and potassium analyses, samples and standards were diluted 1:5 with nanopure water followed by a 1:10 dilution with a 2.5% HNO3 (by volume) matrix solution with 10 ppm yttrium as an internal standard. The two analytical methods for Ca and Mg (titration versus ICP-AES) were compared before coring began. Six water samples were prepared by dilution of IAPSO seawater and analyzed by both the titration and ICP-AES methods for Ca and Mg concentrations. The average percent deviation for Mg concentration between 15 and 54 mM was 1.1% (0.35 mM) and much higher at lower concentrations. For Ca, the average percent deviation between 1 and 10.5 mM was 2.9% (0.1 mM). In addition, at Site U1329 all IW samples were analyzed for Ca and Mg concentrations by both methods. The average deviation for Ca was 15% (0.5 mM) and for Mg it was 4% (1.6 mM). The Ca and Mg concentration data reported in "Interstitial water geochemistry" in the "Site U1329" chapter were obtained by the titration method. At all other sites the reported Ca and Mg data were obtained by ICP-AES. ICP-AES techniques for the minor elements H4SiO4, B, Ba, and Li were modified from those described by Murray et al. (2000) by preparing calibration standards in an acidified (2.5% HNO3 by volume) sodium chloride matrix (35 g NaCl/L). In addition, a 2.5% HNO3 matrix solution with 10 ppm yttrium (1:10) served as an internal standard to dilute standards and acidified IW samples (Mix, Tiedemann, Blum, et al., 2003).
The reproducibility of techniques (Table T2), expressed as 1 |
||

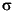 relative standard deviations of the means, was evaluated by replicate analyses of solutions, IAPSO seawater, and/or samples both within a given analytical run and in different analytical runs. The IW data are reported in molar concentration units in the tables of this volume.
relative standard deviations of the means, was evaluated by replicate analyses of solutions, IAPSO seawater, and/or samples both within a given analytical run and in different analytical runs. The IW data are reported in molar concentration units in the tables of this volume.