| IODP Proceedings Volume contents Search | ||

|
||
| Expedition reports Research results Supplementary material Drilling maps Expedition bibliography | ||
|
doi:10.2204/iodp.proc.311.102.2006 Organic geochemistryThe primary focus of the shipboard organic geochemistry program for Expedition 311 was the analysis of volatile hydrocarbon (C1–C5) and nonhydrocarbon (i.e., O2 and N2) gases from headspace (HS) gas samples, void gas samples, gas samples recovered during PCS degassing experiments, and dissociated gas hydrates. We also measured the inorganic carbon (IC), total carbon (TC), and total nitrogen (TN) content of the sediments. Procedures and instruments used during Expedition 311 are described by Pimmel and Claypool (2001) and are generally the same as those used during most recent ODP legs and IODP expeditions. Brief comments on routine sampling and deviations from standard practice are noted below. Gas samplingThe IODP gas sampling protocol for pollution prevention and safety as required by IODP safety regulations was modified to better constrain the concentrations of dissolved gases. We followed the approaches that were employed during previous expeditions with a strong biogeochemical focus, in particular ODP Legs 164 (Hoehler et al., 2000) and 201 (Shipboard Scientific Party, 2003a) and IODP Expeditions 301 (Expedition 301 Scientists, 2005) and 307 (Expedition 307 Scientists, 2006). Samples for HS analysis were collected on the opposite core end facing the IW sample to integrate the IW and gas data sets. The sampling frequency was adjusted to the geochemical redox zonation known from previous studies at the northern Cascadia margin to achieve a high depth resolution at the SMI. The overall IW sampling strategy is explained in "Interstitial water geochemistry." In general, the HS gas sampling protocol only deviated from the IW sampling strategy at depths greater than two cores below the BSR, where one additional HS sample was taken in between the IW samples. Upon core retrieval, a 3 mL sediment sample was collected with a 5 mL cut-off plastic syringe from a freshly exposed end of a core section and was extruded into a 20 mL glass serum vial. For this purpose, the plunger was held at the sediment surface while inserting the barrel to avoid trapping air. After withdrawing the syringe, the plunger was advanced slightly to extrude a small amount of sediment. This excess was removed from the end of the syringe barrel to provide an accurate determination of the sediment volume within the syringe. The samples were immediately sealed with a 10 mm thick septum and metal crimp cap. In the laboratory, 1 mL of saturated NaCl was added to the vials and they were shaken vigorously until the sediment plug disintegrated. Prior to gas chromatograph (GC) analysis, the samples were heated to 60°C for at least 20 min. Air blanks incubated with septum fragments confirmed that no hydrocarbons (C1–C7) were released from the septum. Gas samples from voids caused by gas expansion in the core were collected by piercing the core liner and allowing gas to expand into a 60 mL syringe connected to the penetration tool. Gas hydrate gas samples were collected by placing a small (~5 mL) piece of solid gas hydrate into 60 mL syringes, expelling the air, and allowing the gas hydrate to dissociate. The residual water was preserved for analysis of dissolved constituents (see "Interstitial water geochemistry"). PCS samples were collected in a 60 mL syringe during the degassing experiments (see "Pressure coring"). The gas was transferred into serum vials filled with a saturated NaCl solution by displacing the brine with the gas sample. A few gas samples were analyzed to observe changes in the gas composition during core degassing. In cases where numerous samples from a degassing experiment were analyzed, the average value of samples not contaminated with air or helium is reported. Gas analysisFor HS analysis, 1 to 2 mL of gas was extracted from the vial using a 5 mL plastic syringe. The standard 5 mL gas-tight syringe typically used for IODP GC injections was determined to leak under certain conditions (i.e., blockage of needle with septum), whereas the 5 mL plastic syringe did not. The 1 to 2 mL sample volumes were selected to preserve HS gas for postcruise analyses. Previous ODP legs and IODP expeditions utilized 5 mL injection volumes for the HS analysis. We compared the results from 1 mL and 5 mL injections with the "B" standard and found no significant difference in the detector response for the 1 and 5 mL injection volumes. The HS samples were removed from the 60°C oven immediately before analysis to maintain a constant gas temperature of 60°C during sample injection. A volume of water equivalent to the volume of gas sampled was added to the vials to maintain atmospheric pressure. The vials were then frozen upside down to provide an additional seal during sample storage. For the large-volume void and gas hydrate gas samples, 5 mL of gas was injected directly from the 60 mL syringe through a luer-lock fitting.
Constituents of the HS were analyzed using a Hewlett Packard 6890 Plus (GC3) gas chromatograph equipped with an 8 ft x
Gas hydrate, void gas samples, and selected HS samples were analyzed on the natural gas analyzer (NGA). The NGA system consists of a Hewlett Packard 6890 Plus GC equipped with four different columns and two detectors. Hydrocarbons from methane to hexane were separated using a 60 m x 0.32 mm DB-1 capillary column and analyzed with a FID. The GC oven was heated isothermally at 50°C for 15 min. The hydrocarbons, as well as oxygen, nitrogen, and CO2, were also analyzed with a thermal conductivity detector (TCD). Separation of the compounds was achieved with a multivalve, multicolumn system that includes a 6 inch stainless steel column packed with Poropak T (50/80 mesh), a 3 ft column packed with a 13x molecular sieve, and a 6 ft stainless steel column packed with HayeSep R (acid washed). The precision of analysis for the "B" standard with the NGA was The HS, void, gas hydrate, and PCS gas concentrations are expressed as component parts per million by volume (ppmv) relative to the analyzed gas. To the extent that sampling procedures are uniform, the differences in the HS results reflect differences in the amount of gas remaining in the cores. Major variation in concentrations between the void gas and gas hydrate gas results reflects dilution of the void gas samples with air. The volumetric units of the void gas samples were converted to concentration units (mM) to facilitate comparisons with dissolved IW constituents using
CH4 = ( where
Quantities of methane that remained in solution are minimal (e.g., Duan et al., 1992) and are not accounted for. The internal volumes of 15 representative HS vials were carefully measured beforehand and were determined to average 25.41 ± 0.18 mL. This volume was taken as a constant in calculations of gas concentrations. SedimentsIC concentrations were determined using a Coulometrics 5011 CO2 coulometer. About 10–15 mg of freeze-dried, ground sediment was weighed and reacted with 2N HCl. The liberated CO2 was titrated, and the end-point was determined by a photodetector. Calcium carbonate, expressed as weight percent, was calculated from the IC content, assuming that all evolved CO2 was derived from dissolution of CaCO3, by the following equation: CaCO3 (wt%) = 8.33 x IC (wt%). (2) No correction was made for the presence of other carbonate minerals. TC and TN concentrations were determined using a Carlo Erba 1500 CNS elemental analyzer. About 10 mg of freeze-dried, ground sediment was weighed and combusted at 1000°C in a stream of oxygen. Nitrogen oxides were reduced to nitrogen and the mixture of CO2, nitrogen, and SO2 was separated by GC and detected with a TCD. Total organic carbon concentrations were calculated as the difference between TC and IC concentrations. |
||

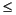 1% for all gases analyzed.
1% for all gases analyzed.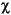 M x Patm x VH)/(R x T x
M x Patm x VH)/(R x T x 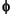 x VS), (1)
x VS), (1)